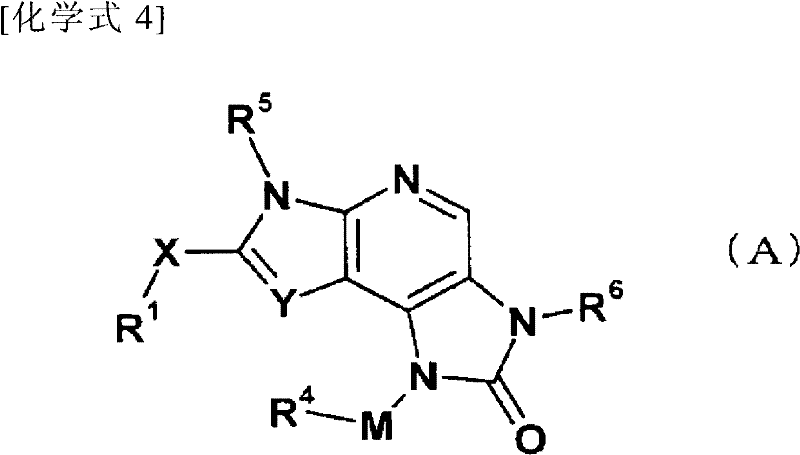
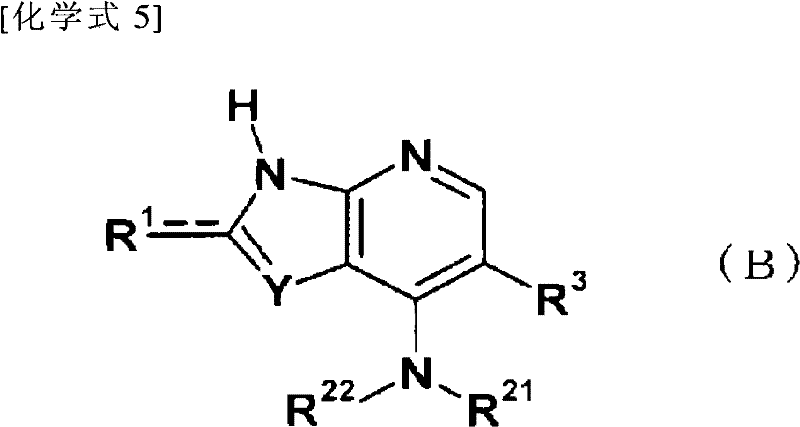
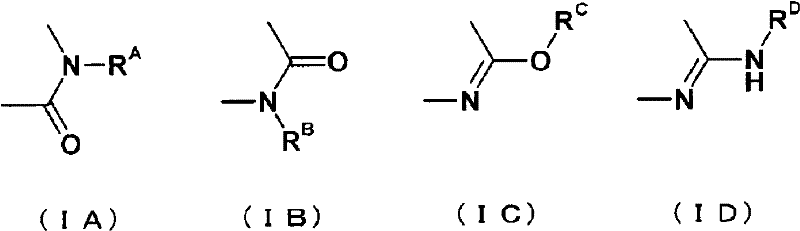
Fused pyrrolopyridine derivative
A kind of technology of pyridine and compound, applied in the field of condensed pyrrolopyridine derivatives, can solve problems such as undisclosed skeleton compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0391] The preparation method of the compound represented by formula (I) will be described in more detail below based on the examples. In addition, the present invention is not limited to the preparation methods shown in the following specific examples and preparation examples, and the compound represented by formula (I) can also be prepared by a combination of these preparation methods or methods obvious to those skilled in the art.
[0392] In addition, the following symbols are used in Examples, preparation examples, and the table|surface mentioned later.
[0393] Pr: Preparation number
[0394] Ex: Example number
[0395] No: compound number
[0396] Data: physical and chemical data
[0397] ESI+: m / z value in ESI-MS (positive ion)
[0398] ESI-: m / z value in ESI-MS (anion)
[0399] APCI+: m / z value in APCI-MS (positive ion)
[0400] NMR-DMSO-d 6 : DMSO-d 6 middle 1 δ(ppm) of H-NMR
[0401] rac-: the racemate of the compound described in the text or in the struct...
preparation example 1
[0423] (methoxymethyl)triphenylphosphine chloride THF (10.6 mL) was added to (1.00 g), and sodium bis(trimethylsilyl)amide (1.07M THF solution, 3.22 mL) was added dropwise under ice cooling, and stirred for 30 minutes. Add dropwise rac-4-{[(3R,4R)-1-benzyl-4-methylpiperidin-3-yl]amino}-1-{[2-(trimethylsilyl ) ethoxy]methyl}-1H-pyrrolo[2,3-b]pyridine-5-carbaldehyde (1.27 g) in THF (10.0 mL), and stirred at room temperature for 4 hours. After adding saturated aqueous ammonium chloride solution to the reaction mixture, the mixture was extracted with EtOAc, and washed with saturated brine. use Na 2 SO 4 After drying the organic layer, it was filtered, and the filtrate was concentrated under reduced pressure. The residue was purified by silica gel column chromatography (EtOAc / Hx=10 / 90~30 / 70) to obtain rac-N-[(3R,4R)-1-benzyl-4-methylpiperidin-3-yl] -5-(2-Methoxyvinyl)-1-{[2-(trimethylsilyl)ethoxy]methyl}-1H-pyrrolo[2,3-b]pyridine-4- Amine (1.34g).
[0424] Compounds of Prep...
preparation example 2
[0426] By continuously carrying out the same method as Preparation Example 1 and Preparation Example 3, the compounds of Preparation Example 2 and Preparation Example 2-1 shown in the following tables were prepared.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com