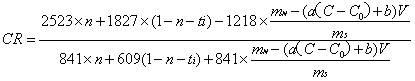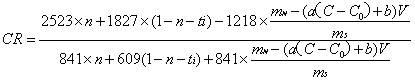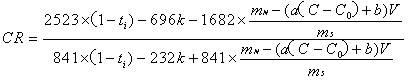Method for measuring molecular ratio of acid KF-NaF-AlF3 electrolyte system
A kf-naf-alf3, measurement method technology, applied in the direction of electrochemical variables of materials, can solve problems such as large errors, obstacles to industrial application of electrolyte systems, inability to accurately control electrolyte components, etc., to achieve accurate and rapid measurement, short measurement time, Easy-to-operate effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Establish a conductivity-NaF standard solution concentration standard curve, the concentration of NaF standard solution is 0.75, 1, 1.25, 1.7, 1.75, 2, 2.25g / L. Weigh 4g of the dried electrolyte sample, add 2g of NaF, grind it in an agate mortar for at least 5min, then sinter in a muffle furnace at 680°C for 30min, take it out and cool it, grind it and weigh it, and then transfer it all into a 250mL beaker. Add 200 mL of deionized water, stir on a magnetic stirrer, and measure the conductivity of the solution. The molecular ratio can be obtained by bringing the content of NaF and other components in the electrolyte into (3) to calculate.
Embodiment 2
[0032] Establish a conductivity-NaF standard solution concentration standard curve, the concentration of NaF standard solution is 1.5, 1.75, 2, 2.25, 2.5, 2.75, 3, 3.25g / L. Weigh 2 g of the dried electrolyte sample, add 1.6 g of NaF, grind it in an agate mortar for at least 5 minutes, then sinter it in a muffle furnace at 600 ° C for 50 minutes, take it out and cool it, grind it and weigh it, and then transfer it all into a 250 mL beaker , add 100 mL of deionized water, stir on a magnetic stirrer, and measure the conductivity of the solution. The molecular ratio can be obtained by bringing the content of NaF and other components in the electrolyte into (3) to calculate.
Embodiment 3
[0034] Establish a conductivity-NaF standard solution concentration standard curve, the concentration of NaF standard solution is 1.0, 1.5, 1.75, 2, 2.25, 2.5, 2.75, 3, 3.25g / L. Weigh 2 g of the dried electrolyte sample, add 1.3 g of NaF, grind it in an agate mortar for at least 5 min, then sinter in a muffle furnace at 680 ° C for 20 min, take it out and cool it, grind it and weigh it, and then transfer it all into a 250 mL beaker , add 100 mL of deionized water, stir on a magnetic stirrer, and measure the conductivity of the solution. The molecular ratio can be obtained by bringing the content of NaF and other components in the electrolyte into (3) to calculate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com