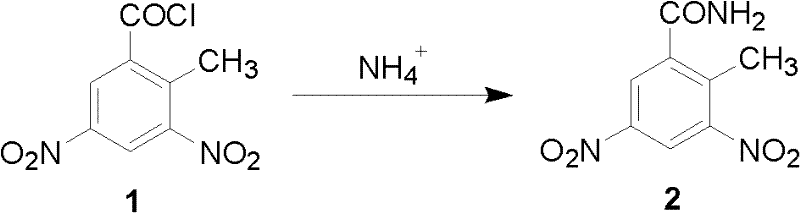
Preparation method of 3, 5-binitro-2-methyl benzamide
A technology of methyl benzamide and methyl benzoyl chloride, which is applied in 3 fields, can solve the problems of high cost, excess, and ammonia volatilization, and achieve the effects of cost saving, quantitative control, and avoiding volatilization problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] The molar ratio of 3,5-dinitro-2-methylbenzoyl chloride to ammonium chloride is 1:1.
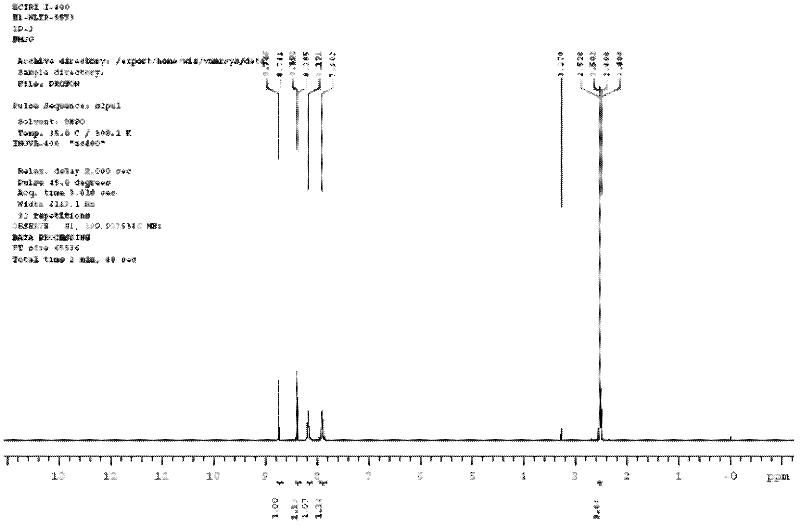
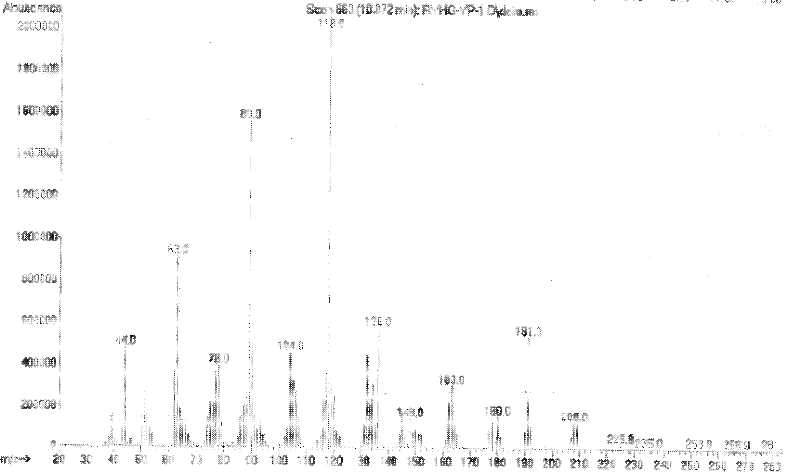
[0022] In a 1000mL four-neck flask equipped with a reflux condenser and a thermometer, add 21.4g of ammonium chloride and 200ml of water, start stirring, and raise the temperature to 35°C within 30 minutes to completely dissolve the ammonium chloride, slowly add 3,5 -Dinitro-2-methylbenzoyl chloride 97.8g, dripped in 1 hour, kept the reaction temperature at 35°C, and continued to react for 2 hours. After the reaction, the reaction solution was filtered, the filter cake was washed with water, filtered, and filtered The cake was dried at 100° C. to obtain 81 g of 3,5-dinitro-2-methylbenzamide (Globalin), with a yield of 90%. The H NMR spectrum is shown in figure 1 shown, and the mass spectrum see figure 2 shown.
Embodiment 2
[0024] The molar ratio of 3,5-dinitro-2-methylbenzoyl chloride to ammonium chloride is 1:3.
[0025] In a 1000mL four-neck flask equipped with a reflux condenser and a thermometer, add 64.2g of ammonium chloride and 600ml of water, start stirring, and raise the temperature to 35°C within 30 minutes to completely dissolve the ammonium chloride, slowly add 3, 97.8g of 5-dinitro-2-methylbenzoyl chloride was dropped in 1 hour, and the reaction temperature was kept at 35°C, and the reaction was continued for 2 hours. After the reaction, the reaction solution was filtered, and the filter cake was washed with water and filtered. The filter cake was dried at 100° C. to obtain 85.5 g of globulin, with a yield of 95%.
Embodiment 3
[0027] Feeding ratio 3, the material ratio of 5-dinitro-2-methylbenzoyl chloride to ammonium chloride is 1:3, and the concentration of ammonium chloride aqueous solution is about 25%.
[0028] In a 1000mL four-neck flask equipped with a reflux condenser and a thermometer, add 64.2g of ammonium chloride and 200ml of water, start stirring, and raise the temperature to 35°C within 30 minutes to completely dissolve the ammonium chloride, slowly add 3, 97.8g of 5-dinitro-2-methylbenzoyl chloride was dropped in 1 hour, and the reaction temperature was kept at 35°C, and the reaction was continued for 2 hours. After the reaction, the reaction solution was filtered, and the filter cake was washed with water and filtered. The filter cake was dried at 100° C. to obtain 89.1 g of globulin, with a yield of 99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com