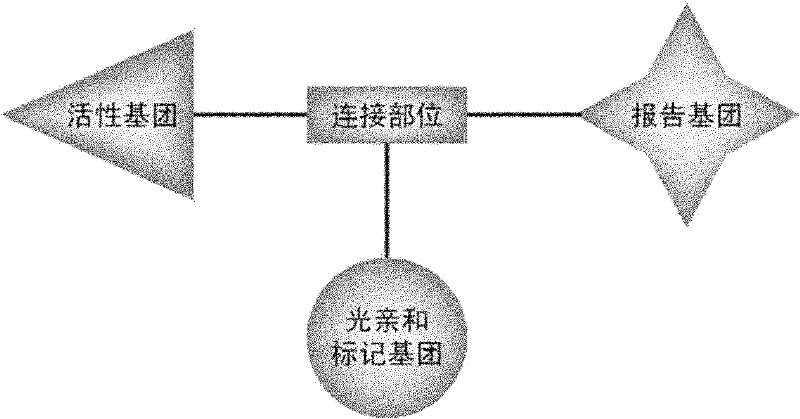
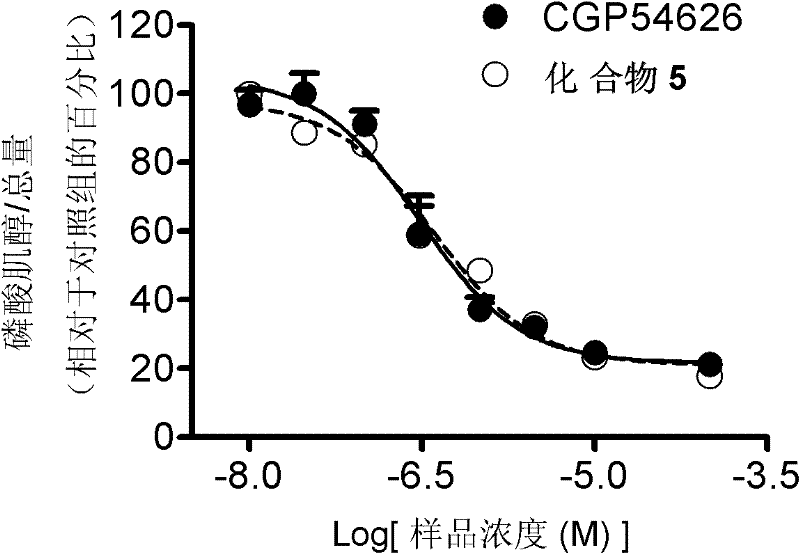
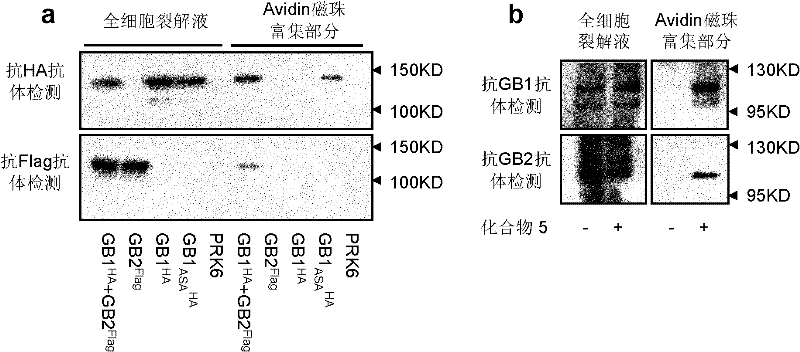
First glass photoaffinity labeling difunctional probe molecule and preparation method and application thereof
A probe molecule, labeled double technology, applied in live or field
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0069] Preparation Example 1——Preparation of Compound 5
[0070] Preparation of compound 3
[0071]
[0072] Compound 1 (53mg, 0.12mmol, preparation reference Bioorg.Med.Chem., 1999, 7:2697-2704) was dissolved in 3mL of methanol, compound 2 (71mg, 0.13mmol, preparation reference J.Med.Chem., 2008 , 11:3057-3060) and i-Pr 2 NEt (92μl, 0.52mmol), the system was stirred overnight at room temperature in the dark, the solvent was spin-dried under reduced pressure and then purified by silica gel column chromatography, CHCl 3 :MeOH:H 2 Gradient elution of O=2:1:0-2:1:0.2 gave 68 mg of a colorless oily substance with a yield of 71.3%.
[0073] 1 HNMR (δ, ppm, CD 3 OD+CDCl 3 ): 1.42-1.90 (10H, m), 1.72 (3H, d, J=6.3Hz), 2.89-3.01 (2H, m), 3.35-3.38 (4H, m), 3.53 (2H, m), 3.63 ( 2H, m), 3.69(2H, m), 3.85(2H, s), 3.89(2H, m), 4.24(1H, m), 4.35(2H, m), 4.37(1H, q, J=6.9Hz ), 6.80(1H, s), 6.98(1H, d, J=8.4Hz), 7.47(1H, t, J=7.5Hz), 7.57(1H, d, J=7.2Hz), 7.99-8.08(3H , m).HR-ESI...
preparation Embodiment 2
[0078] Preparation Example 2——Preparation of Compound 7
[0079] Preparation of compound 6
[0080]
[0081] Rodamine B (500mg, 1.04mmol) was dissolved in dry DCM (25mL), DCC (430mg, 2.08mmol), HOBt (282mg, 2.08mmol) and propargylamine (100μL, 1.57mmol) were added sequentially under stirring. The reaction solution was stirred at room temperature for 18 h, and the solvent was spin-dried under reduced pressure, and purified by silica gel column chromatography, eluted with PE:EA=4:1 to obtain 311 mg of a white solid with a yield of 61.8%.
[0082] 1 HNMR (δ, ppm, CDCl 3 ): 1.15 (12H, t, J = 6.9Hz), 1.77 (1H, t, J = 2.4Hz), 3.34 (8H, q, J = 6.9Hz), 3.95 (2H, d, J = 2.4Hz), 6.28(2H, d, J=9.0Hz), 6.40(2H, s), 6.48(2H, d, J=9.0Hz), 7.10(1H, m), 7.43(2H, m), 7.93(1H, m ). 13 CNMR (δ, ppm, CDCl 3 )12.72, 28.62, 44.54, 64.90, 70.15, 78.40, 97.91, 105.20, 108.11, 123.10, 123.90, 128.11, 129.21, 130.54, 132.75, 148.97, 153.60, 153.88, 167.48
[0083] Preparation of compound 7
...
preparation Embodiment 3
[0087] Preparation Example 3——Preparation of Compound 9
[0088] Preparation of compound 8
[0089]
[0090] Protected HA (200 mg, 0.062 mmol) was dissolved in dry DCM (3 mL), EDC (23.8 mg, 0.125 mmol), Et 3 N (13 mg, 0.138 mmol), DMAP (1.5 mg, 0.0125 mmol) and propargylamine (17.2 mg, 0.312 mmol). The reaction solution was stirred at room temperature for 18 h, and the solvent was spin-dried under reduced pressure, and purified by silica gel column chromatography, CHCl 3 :MeOH=25:1, 78 mg of light yellow solid was eluted, and the yield was 76.2%.
[0091] 1 HNMR (δ, ppm, CDCl 3 ): 0.94 (3H, d, J=6.6Hz), 0.98-1.06 (6H, m), 1.15-1.30 (27H, m), 1.33-1.48 (18H, m), 1.70-2.30 (9H, m), 2.52-3.33(11H, m), 3.45-3.80(5H, m), 3.85-4.70(12H, m), 4.75-4.85(1H, m), 5.69(1H, t, J=6.3Hz), 5.78( 1H, t, J=7.2Hz), 6.76-7.00 (5H, m), 7.00-7.12 (3H, m), 7.12-7.22 (2H, m), 7.22-7.45 (5H, m), 7.45-7.67 ( 3H, m), 7.75 (2H, dd, J=10.8, 8.1Hz), 7.90 (1H, dd, J=15.6, 8.1Hz).ESIMS: m / z=843.6(M...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com