Spectinamides as anti-tuberculosis agents
A spectinomycin, halogen technology, applied in the direction of antibacterial drugs, medical preparations containing active ingredients, drug combinations, etc., can solve the problems of limited treatment options, increased number, patient non-compliance, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0792] Synthesis of Spectinomycin Analogs
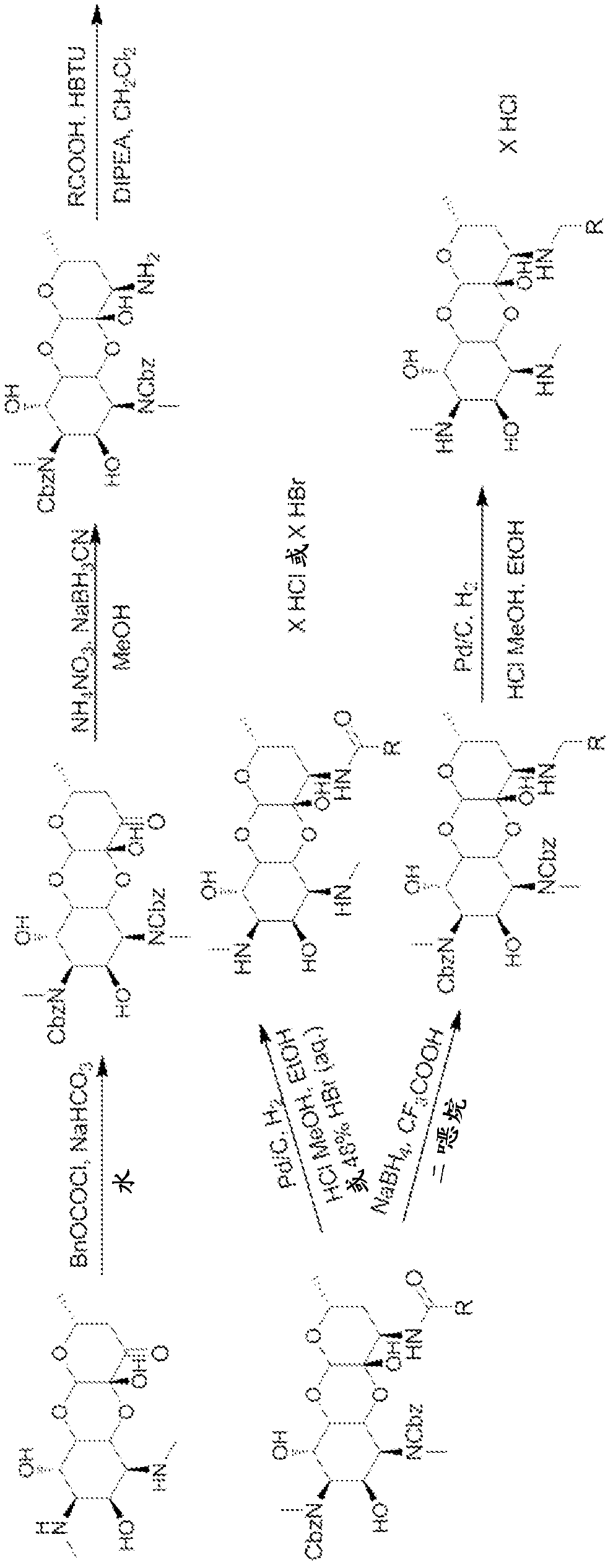
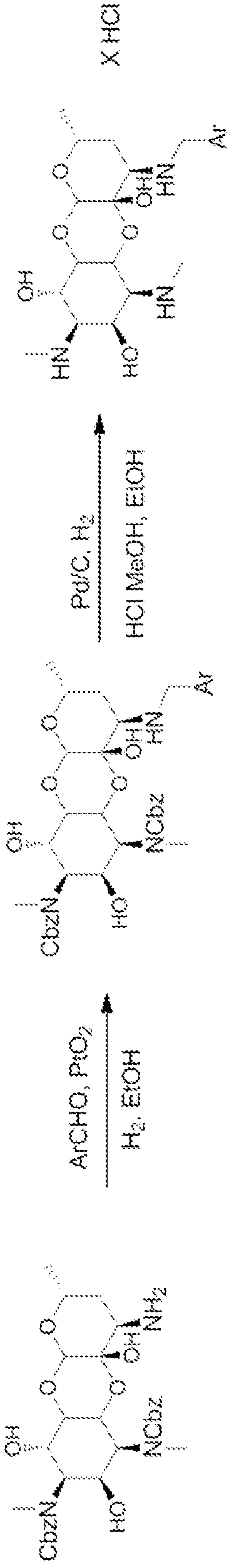
[0793] General Methods: The 3'-deoxy3'-acylamino and 3'-deoxy3'(R)-alkylamino spectinomycins disclosed in the present invention were synthesized according to methods similar to those previously described. see Woitun et al. J. Antibiot (Tokyo), 34(1), 22-27(1981); and Maier et al. , J. Antibiot (Tokyo), 34(1), 16-21 (1981). 1,3-Dibenzyloxycarbonyl-3′(R)-aminospectinomycin from spectinomycin dihydrochloride pentahydrate as figure 1 The synthesis shown is in two steps. First, apply benzyl chloroformate and NaHCO in water 3 , the methyl secondary amine in ring A was protected as benzyl carbamate with carboxybenzyl (CBz). The protected intermediate was then reductively aminated with ammonium nitrate and sodium cyanoborohydride in methanol to afford the 3'-deoxy-3'-amino derivative.
[0794] The amines were then used in the synthesis of the target 3'-acylaminospectinomycin derivatives by coupling them to various acids by using H...
Embodiment 2
[0870] General in vitro and in vivo methods
[0871] MIC determination: application according to Clinical Laboratory Standards Institute (Clinical Laboratory Standards Institute) (CLSI; National, C.F.C.L.S., Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically-Seventh Edition: Approved Standard M7-A7, CLSI, Wayne, Pennsylvania, U.S.A., 2008) was used to measure MIC by the micro broth dilution method, and the readings were checked visually. 2-fold serial dilutions of antibiotics in 100 μL of appropriate broth medium were first prepared in 96-well round bottom microtiter plates (Nalge Nunc International, Rochester, New York, USA). Add an equal volume (100 μL) of about 10 5 Bacterial broth inoculum in cfu / mL of bacteria to give a final drug concentration starting at 200 μg / mL and the plates were incubated aerobically at 37°C. M. tuberculosis and M. bovis BCG microtiter plates were incubated for 7 days, while the other strains were incuba...
Embodiment 3
[0883] Antituberculous activity
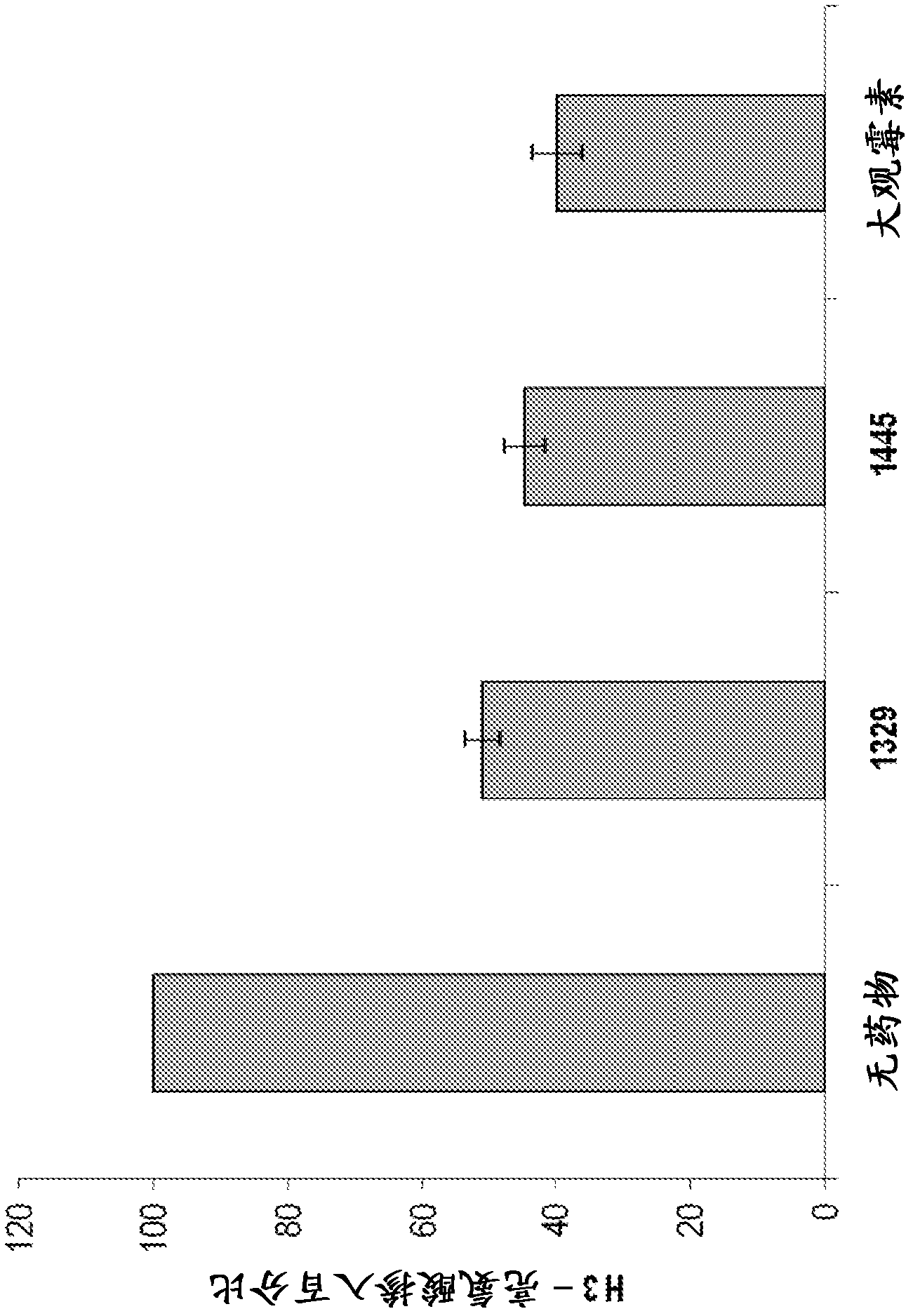
[0884] The anti-tuberculosis activity of spectinomycin analogs against Mycobacterium tuberculosis H37Rv was determined in Middlebrook 7H9 supplemented with 10% ADC medium by dilution in drug microbroth in 96-well plates. Plates were incubated at 37°C for 7 days and then read visually for growth inhibition according to a previously described method. See for example Hurdle et al. , J. Antimicrob. Chemother, 62(5), 1037-1045 (2008). The results are shown in Table 1 and Table 2.
[0885] Table 1.3'-deoxy3'(R)-acylamino spectinomycin anti-tuberculosis activity
[0886]
[0887]
[0888]
[0889]
[0890] Table 2.3'-Deoxy 3'(R)-Alkylamino Spectinomycin Anti-tuberculosis Activity
[0891]
[0892] Multiple compounds showed good anti-tuberculosis MIC values, many of which had better anti-tuberculosis activity compared to spectinomycin. The structure-activity relationship of this series is very tight in terms of structural change...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com