Triterpene compound and application thereof in preparation of anti-complementary medicament
A technology of triterpene compounds and compounds, which is applied in the field of new applications in the preparation of anti-complement drugs, and can solve problems such as Prunella vulgaris compounds that have not yet been seen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1. Preparation of triterpenoids
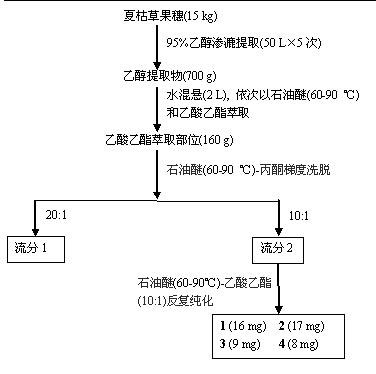
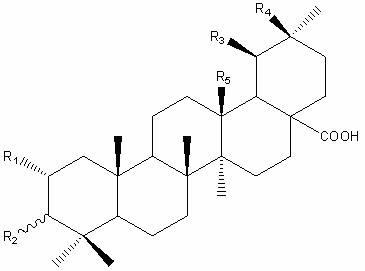
[0022] Take 15 kg of Prunella vulgaris fruit ears, cold-soak with 95% ethanol at room temperature (50 L × 5 times), combine the extracts and concentrate until there is no alcohol smell, and dilute the extract (700 g) to 2 L with water; -90 °C) and ethyl acetate extraction (each 2 L × 3 times), the ethyl acetate extracts were combined and concentrated to dryness to obtain 160 g of ethyl acetate extract. The ethyl acetate extraction part was separated by silica gel column chromatography, and petroleum ether (60-90 °C), petroleum ether (60-90 °C)-acetone, acetone gradient elution, the obtained petroleum ether (60-90 °C)- Acetone (10:1) fractions were purified by repeated silica gel column chromatography using petroleum ether (60-90 °C)-ethyl acetate as eluent to obtain compound 1 (16 mg), compound 2 (17 mg), 3 (9 mg) and 4 (8 mg). Their structures were analyzed by spectroscopic methods and identified as 2α,3α-dihydroxy-olean-1...
Embodiment 2
[0028] Example 2. Anti-complement classical pathway test in vitro
[0029] Take 0.1 ml of complement (guinea pig serum), add barbiturate buffer solution (BBS) to prepare a 1:5 solution, and double-dilute with BBS to 1:10, 1:20, 1:40, 1:80, 1: 160, 1:320 and 1:640 solutions. Dissolve 0.1 ml of 1:1000 hemolysin, various concentrations of complement and 2% sheep red blood cells (SRBC) in 0.3 ml BBS, mix well, put in a low-temperature high-speed centrifuge at 5000 rpm, 4 oC Centrifuge for 10 min under conditions. Take 0.2 ml of the supernatant from each tube in a 96-well plate, and measure the absorbance at 405 nm. A full hemolysis group (0.1 ml 2% SRBC dissolved in 0.5 ml triple distilled water) was also set up in the experiment. The absorbance of three-distilled water lysed blood vessels was used as the standard of total hemolysis, and the hemolysis rate was calculated. Taking the dilution of complement as the X-axis, the percentage of hemolysis caused by each dilution of co...
Embodiment 3
[0031] Example 3. Anti-complement alternative pathway test in vitro
[0032] Take 0.2 ml of complement (human serum), add AP diluent to prepare a 1:5 solution, and double-dilute to 1:10, 1:20, 1:40, 1:80, 1:160, 1:320 and 1:640 solution. Take 0.15 ml of complement of each concentration, 0.15 ml of AP diluent and 0.20 ml of 0.5% rabbit erythrocyte (RE), mix well, place in a low-temperature high-speed centrifuge at 5000 rpm and 4 oC for 10 min in a water bath at 37 oC . Take 0.2 ml of the supernatant from each tube in a 96-well plate, and measure the absorbance at 405 nm. A full hemolysis group (0.20 ml 0.5% RE dissolved in 0.3 ml triple distilled water) was also set up in the experiment. The absorbance of three-distilled water lysed blood vessels was used as the standard of total hemolysis, and the hemolysis rate was calculated. Taking the dilution of complement as the X-axis, the percentage of hemolysis caused by each dilution of complement is plotted as the Y-axis. The l...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com