A kind of lithium ion battery manganese cobalt lithium oxide cathode material and preparation method thereof
A battery manganese cobalt lithium, positive electrode material technology, applied in the direction of battery electrodes, cobalt oxide/cobalt hydroxide, circuits, etc., can solve the electrochemical performance deterioration of positive electrode materials, poor repeatability of the coprecipitation process, and is not conducive to product uniformity and other problems , to achieve excellent electrochemical performance, easy to control, and save production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1: Li 1.2 Mn 0.4 Co 0.4 O 2 Positive electrode material
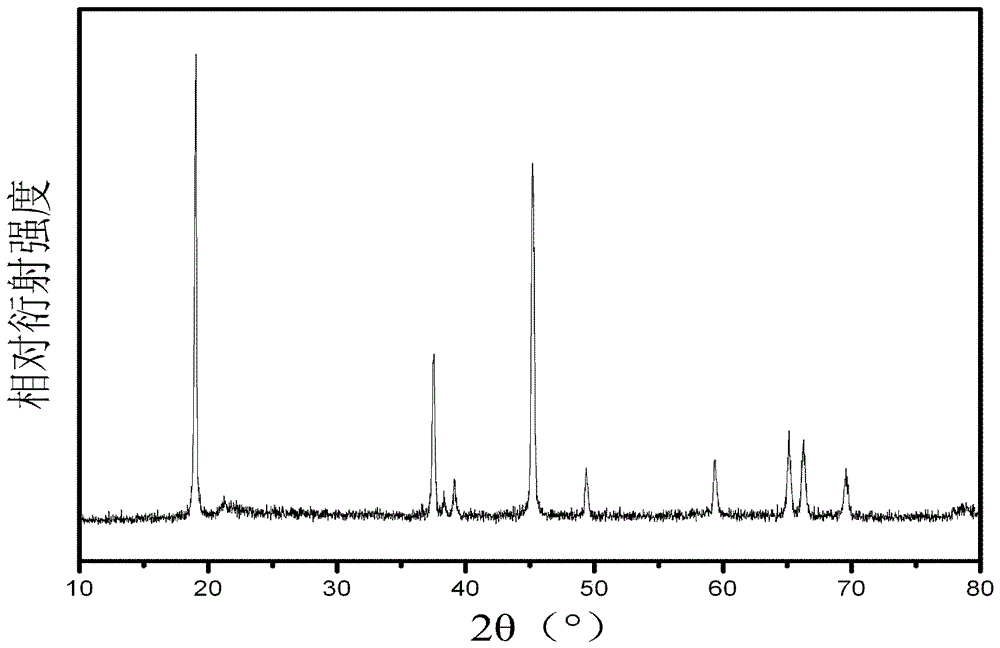
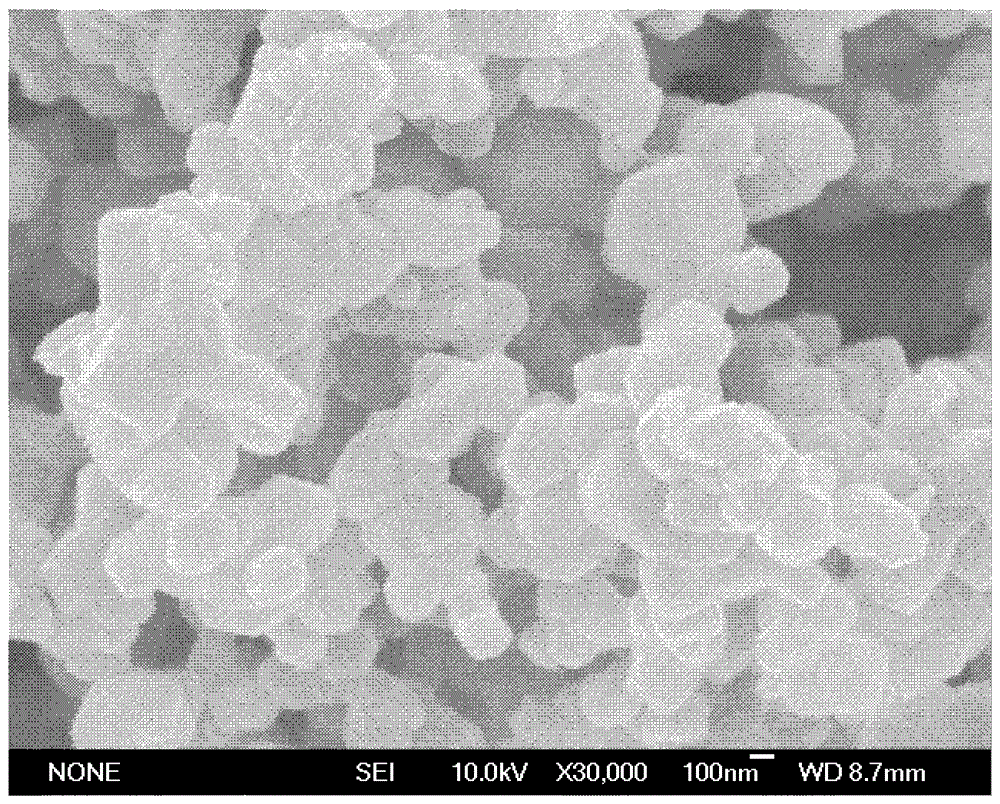
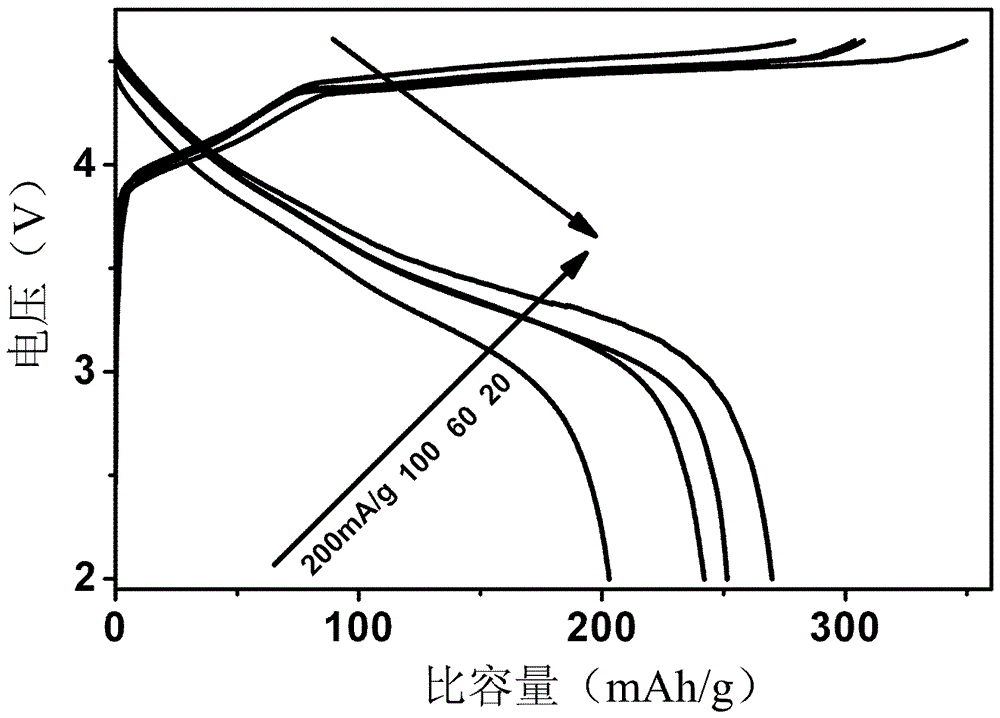
[0024] Taking lithium acetate, manganese acetate, cobalt acetate and oxalic acid as starting materials, dissolving 0.9804g manganese acetate, 0.9964g cobalt acetate and 1.2855g lithium acetate in absolute ethanol to obtain a metal salt solution of ethanol; 2.1634g of excess 20% oxalic acid was dissolved in absolute ethanol; the ethanolic solution of oxalic acid was added to the metal salt solution of ethanol, stirred for 1 hour, filtered with suction, dried at 100°C for 3 hours, and ground into powder and put into a crucible; The furnace was heated to 900°C for 12h at a heating rate of 4°C / min, and then annealed at room temperature to obtain Li. 1.2 Mn 0.4 Co 0.4 O 2 powder material. The XRD test results of the powder material show that the synthesized powder has a layered rock-salt structure (R3m), such as figure 1 shown. The SEM test results of the powder materials show that the synthesized po...
Embodiment 2
[0025] Example 2: Li 1.033 Mn 0.067 Co 0.9 O 2 Positive electrode material
[0026] Taking lithium acetate, manganese acetate, cobalt acetate and oxalic acid as starting materials, dissolving 0.3270g manganese acetate, 4.4836g cobalt acetate and 2.5305g lithium acetate in absolute ethanol to obtain a metal salt solution of ethanol; 4.8021g of excess 20% oxalic acid was dissolved in absolute ethanol; the ethanolic solution of oxalic acid was added to the ethanolic metal salt solution, stirred for 1 hour, filtered with suction, dried at 100°C for 3 hours, ground into powder and put into a crucible; The furnace was heated to 900°C for 12h at a heating rate of 4°C / min, and then annealed at room temperature to obtain Li. 1.033 Mn 0.067 Co 0.9 O 2 powder material. The XRD test results of the powder material show that the synthesized powder has a layered rock-salt structure (R3m). The SEM test results of the powder materials show that the synthesized powder particles are uni...
Embodiment 3
[0027] Example 3: Li 1.1 Mn 0.2 Co 0.7 O 2 Positive electrode material
[0028] Taking lithium acetate, manganese acetate, cobalt acetate and oxalic acid as starting materials, 0.9804g manganese acetate, 3.4873g cobalt acetate and 2.4689g lithium acetate are dissolved in absolute ethanol to obtain a metal salt solution of ethanol; 6.3842g of excess 20% oxalic acid was dissolved in absolute ethanol; the ethanolic solution of oxalic acid was added to the metal salt solution of ethanol, stirred for 1 hour, filtered with suction, dried at 100°C for 3 hours, ground into powder and put into a crucible; The furnace was heated to 900°C for 12h at a heating rate of 4°C / min, and then annealed at room temperature to obtain Li. 1.1 Mn 0.2 Co 0.7 O 2 powder material. The XRD test results of the powder material show that the synthesized powder has a layered rock-salt structure (R3m). The SEM test results of the powder materials show that the synthesized powder particles are uniform...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com