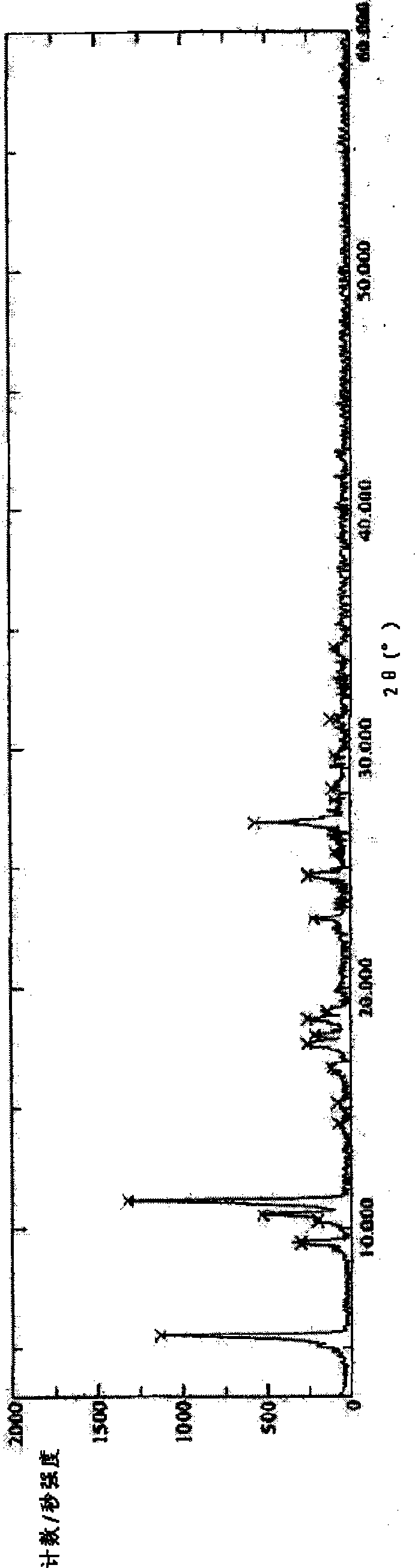
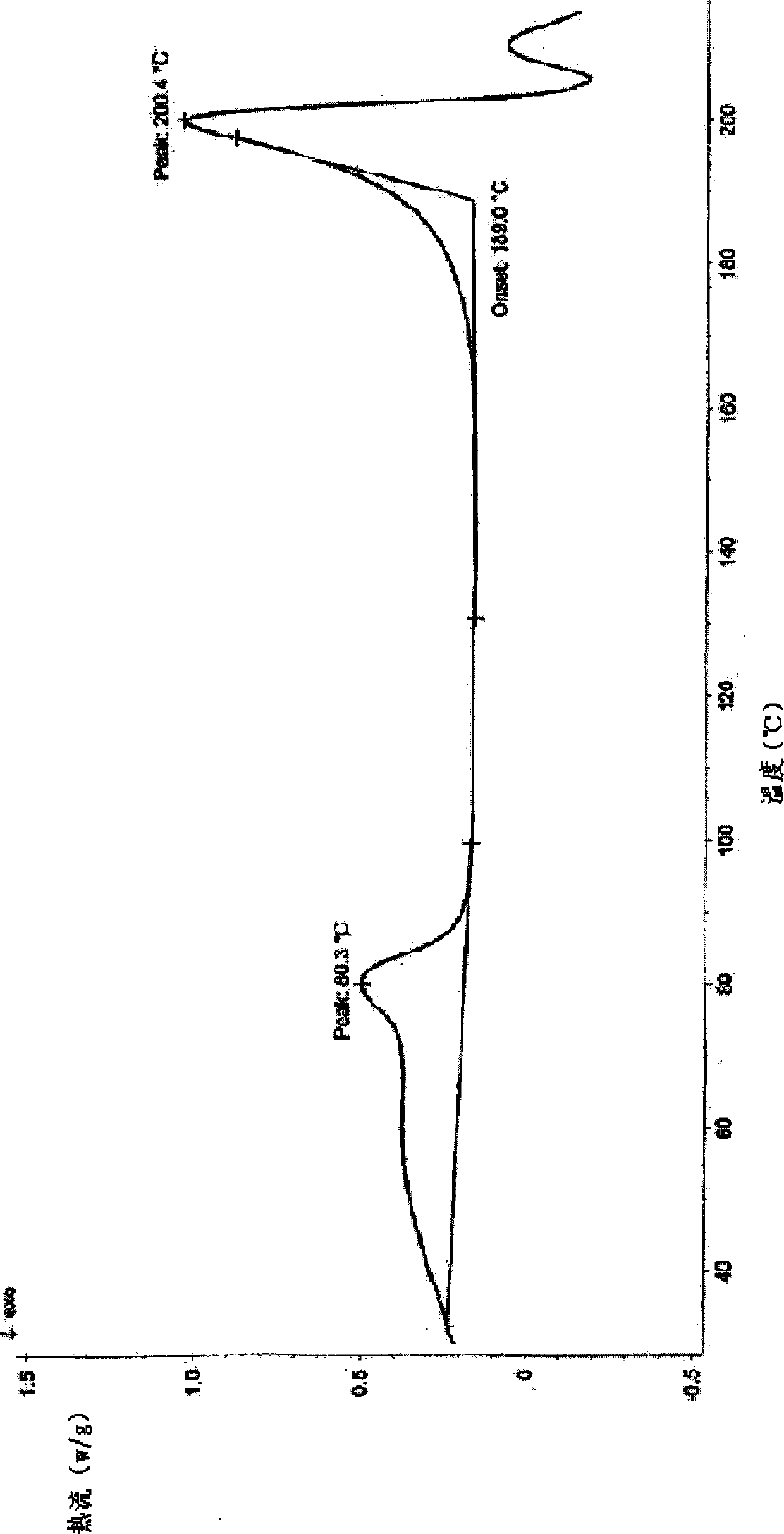
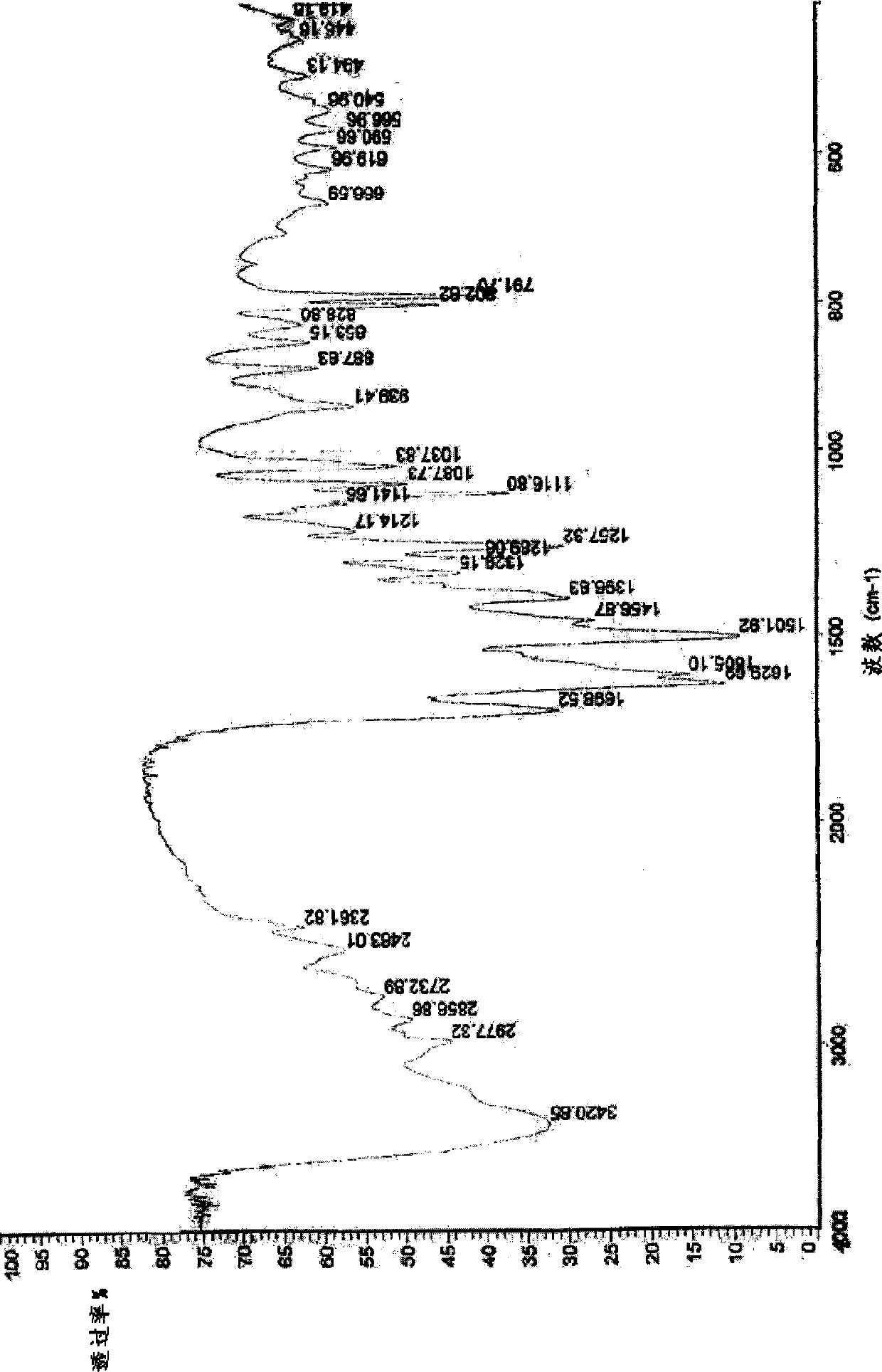
Lactic levorotatory ulifloxacin crystal, and preparation method and application thereof
A technology of ulifloxacin and lactic acid, which is applied in the field of Lactate L-ulifloxacin crystals and its preparation and application, can solve the problems of insufficient water content, unstable properties, difficult operation, etc., achieve good clinical application potential, and simple preparation method , good reproducible effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0057] Specifically, the preparation method adopted in the present invention can exemplarily include:
[0058] 1. (S)-6-fluoro-1-methyl-4-oxo-7-(1-piperazinyl)-1H,4H-[1,3]thiazetidine[3,2 -a] the preparation of quinoline-3-carboxylic acid (levo-ulifloxacin)
[0059] 105 grams of racemic ulifloxacin were dissolved in 1500 ml of dimethyl sulfoxide, and a dimethyl sulfoxide solution of 405 ml of D-tartaric acid (27 grams) was added dropwise with stirring, turbidity and precipitation occurred, and stirred at room temperature for 20 hours; Filtration, the obtained solid was dried under vacuum to obtain 86 g, and the solid was recrystallized and purified in dimethyl sulfoxide to obtain (S)-6-fluoro-1-methyl-4-oxo-7-(1- Piperazinyl)-1H, 4H-[1,3]thiazetidino[3,2-a]quinoline-3-carboxylic acid-D-tartrate 37 grams. Add this salt into water to form a suspension, adjust the pH value to 7-8 with 2% NaOH aqueous solution under stirring, and filter and dry the precipitate to obtain (S)-6-fl...
Embodiment 1
[0070] Preparation of L-ulifloxacin Lactate Crystal A (prepared with reference to the method of Chinese invention patent application CN200810027211.9)
[0071]At room temperature 20°C, add 30ml of water to the reaction flask, add 2.1 grams of lactic acid while stirring, and then add 5 grams of (S)-6-fluoro-1-methyl-4-oxo-7-(1-piperazine base)-1H, 4H-[1,3]thiazetidino[3,2-a]quinoline-3-carboxylic acid, after stirring for 60 minutes to obtain a substantially clear solution, adding (S)-6 -Fluoro-1-methyl-4-oxo-7-(1-piperazinyl)-1H,4H-[1,3]thiazetidino[3,2-a]quinoline-3 5% of the weight of the carboxylic acid was decolorized by activated carbon for 30 minutes and then filtered, and the filtrate was added dropwise with 200 ml of absolute ethanol within 1 hour under stirring, at this time, a solid was precipitated, and the stirring was continued for 2 hours, the solid was filtered, and vacuum-dried at 60°C to obtain 4.0 grams Lactic acid (S)-6-fluoro-1-methyl-4-oxo-7-(1-piperazinyl...
Embodiment 2
[0074] Preparation of L-ulifloxacin Lactate Crystal B
[0075] At room temperature, add 150ml of water to the reaction flask, add 14.7 grams of lactic acid under stirring, and then add 35 grams of (S)-6-fluoro-1-methyl-4-oxo-7-(1-piperazinyl) -1H, 4H-[1,3]thiazetidino[3,2-a]quinoline-3-carboxylic acid, add (S)-6-fluoro-1-methyl-4-oxo -7-(1-piperazinyl)-1H, 4H-[1,3]thiazetidin[3,2-a]quinoline-3-carboxylic acid weight of 5% activated carbon 50 ℃ insulation Stir for 30 minutes to decolorize and filter, evaporate the filtrate to remove the solvent under reduced pressure, add 100ml of ethanol, filter, collect the solid and dry it in vacuum at 60°C to obtain 34 grams of lactic acid (S)-6-fluoro-1-methyl-4-oxo-7 Crude -(1-piperazinyl)-1H,4H-[1,3]thiazetidino[3,2-a]quinoline-3-carboxylic acid.
[0076] 5 grams of lactic acid (S)-6-fluoro-1-methyl-4-oxo-7-(1-piperazinyl)-1H,4H-[1,3]thiazetidine[3, 2-a] Quinoline-3-carboxylic acid crude product, 0.25 g of activated carbon, 100 ml of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com