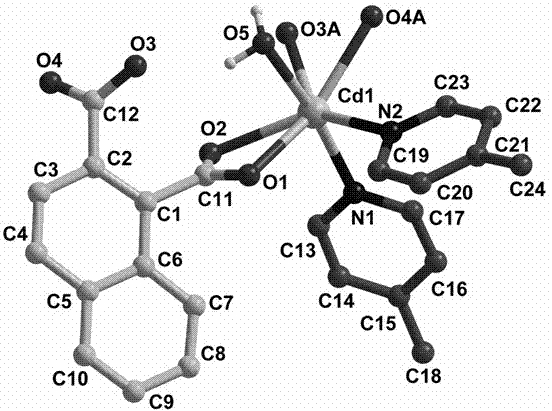
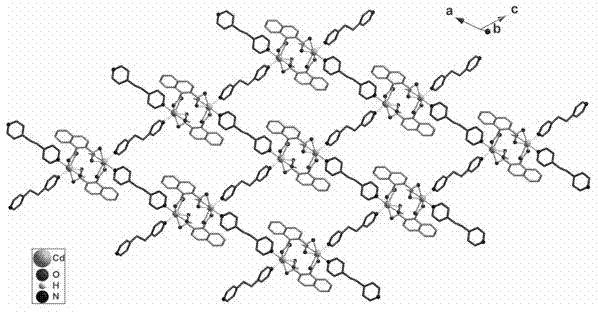
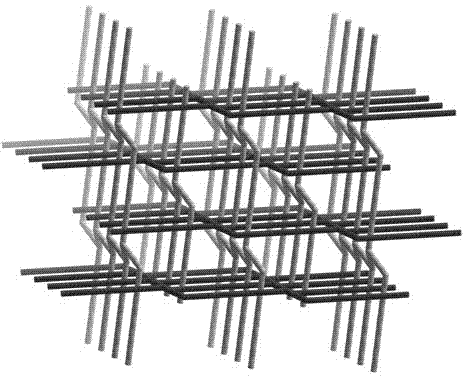
1,2-naphthalene diacid containing cadmium coordination complex and preparation method thereof
A technology for cadmium naphthalene diacid and complexes, which is applied in the fields of cadmium organic compounds, chemical instruments and methods, luminescent materials, etc., and can solve the problem of unreported research on metal complexes, achieving high yield and good reproducibility , good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] 1,2-Naphthalic anhydride (0.075 mmol, 15.4 mg), 1,2-bis(4-pyridyl)ethane (0.1 mmol, 18.4 mg) and cadmium acetate (0.2 mmol, 50.0 mg) were dissolved in water (10 mL) was stirred for several minutes and then sealed into a 25ml hydrothermal reaction kettle. Then the reaction mixture was heated at 10 °C per hour to 120 °C, maintained at this temperature for 3 days, and then lowered to room temperature to obtain colorless blocky crystals, which were separated, washed and dried in turn to obtain The target product, the yield is about 45%. The main infrared absorption peaks are: 3378, 3061m, 2927m, 2360W, 1613M, 1567S, 1501W, 1469W, 1418VS, 1353m, 1321W, 1223M, 1066M, 1020W, 874W, 816W, 778W, 662W, 550W, 535W, 550W, 550W, 550W, 550W, 5535W, 550W, 535W, 550W, 5535W, .
Embodiment 2
[0027] 1,2-Naphthalic anhydride (0.075 mmol, 15.4 mg), 1,2-bis(4-pyridyl)ethane (0.1 mmol, 18.4 mg) and cadmium acetate (0.2 mmol, 50.0 mg) were dissolved in water ( 12 mL) was stirred for several minutes and then sealed into a 25 mL hydrothermal reaction kettle. Then the reaction mixture was heated at 10°C per hour to 130°C, maintained at this temperature for 3 days, and then lowered to room temperature to obtain a colorless block crystal, which was separated, washed and dried in turn to obtain The target product, the yield is about 55%. The main infrared absorption peaks are: 3378, 3061m, 2927m, 2360W, 1613M, 1567S, 1501W, 1469W, 1418VS, 1353m, 1321W, 1223M, 1066M, 1020W, 874W, 816W, 778W, 662W, 550W, 535W, 550W, 550W, 550W, 550W, 5535W, 550W, 535W, 550W, 5535W, .
Embodiment 3
[0029] 1,2-Naphthalic anhydride (0.075 mmol, 15.4 mg), 1,2-bis(4-pyridyl)ethane (0.1 mmol, 18.4 mg) and cadmium acetate (0.2 mmol, 50.0 mg) were dissolved in water ( 15 mL) was stirred for several minutes and then sealed into a 25 mL hydrothermal reaction kettle. Then the reaction mixture was heated at 10 °C per hour to 140 °C, maintained at this temperature for 3 days, and then lowered to room temperature to obtain colorless blocky crystals, which were separated, washed and dried in turn to obtain The target product, the yield is about 65%. The main infrared absorption peaks are: 3378, 3061m, 2927m, 2360W, 1613M, 1567S, 1501W, 1469W, 1418VS, 1353m, 1321W, 1223M, 1066M, 1020W, 874W, 816W, 778W, 662W, 550W, 535W, 550W, 550W, 550W, 550W, 5535W, 550W, 535W, 550W, 5535W, .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com