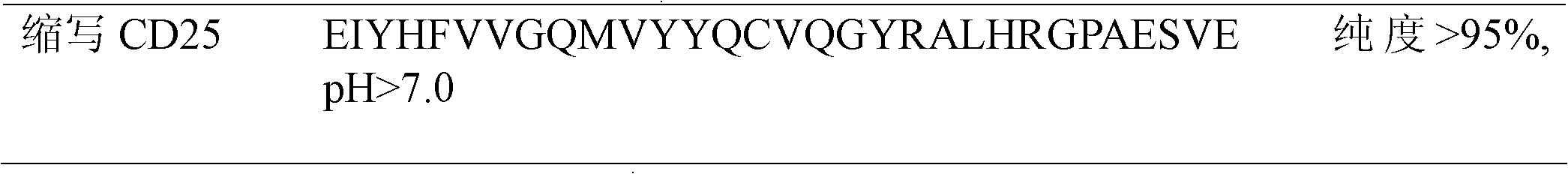Tumor marker CD25 autoantibody and application thereof
A technology of tumor markers and autoantibodies, which is applied in the field of preparation of early diagnosis reagents for tumors and development of targeted drugs for the treatment of tumors, can solve problems such as low sensitivity and poor specificity, and achieve high sensitivity and high specificity effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The binding process of CD25 antigen polypeptide to serum and plasma IgG
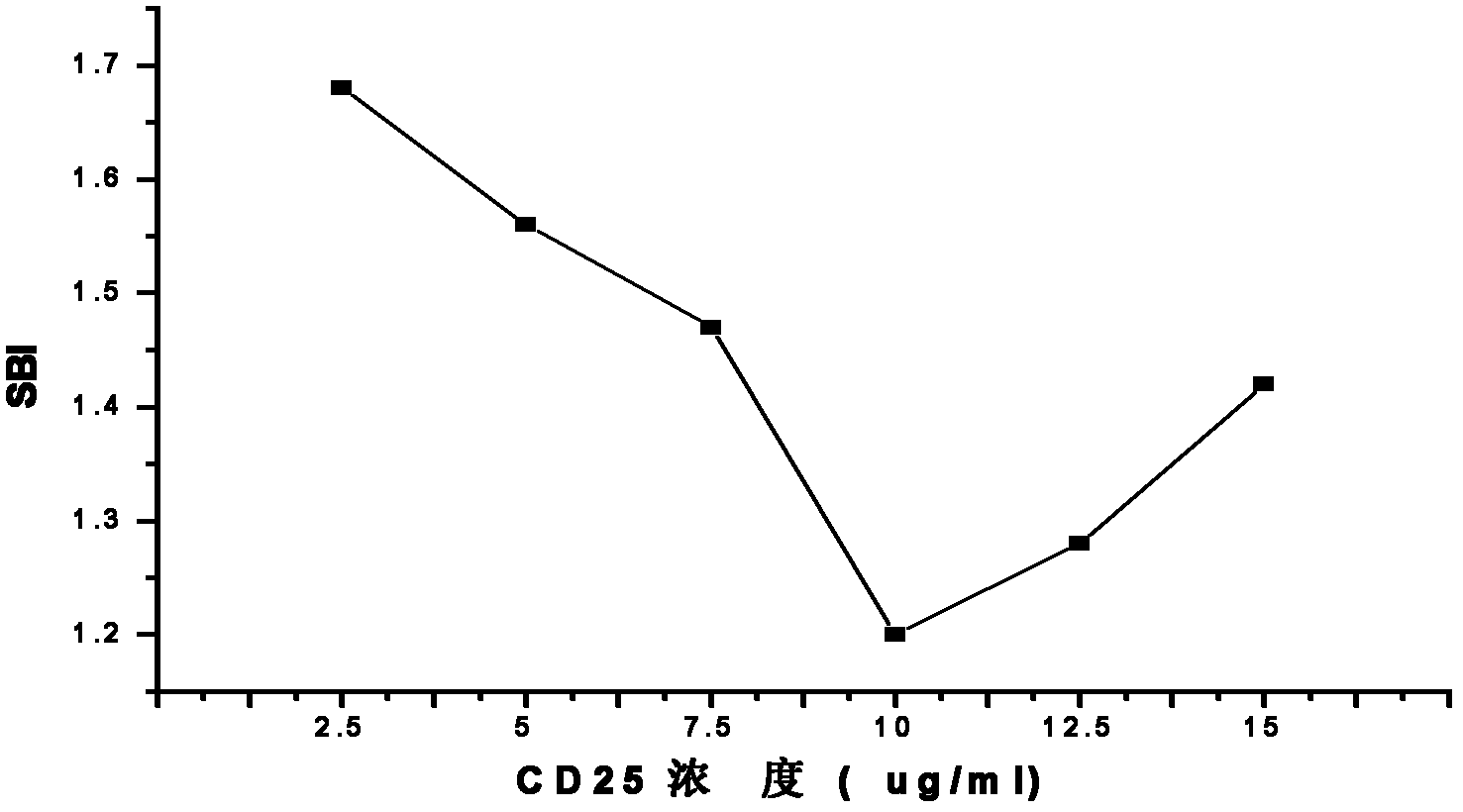
[0038] It can be seen from Figure 1 that when the CD25 concentration is 5-10 μg / ml, the SBI value gradually decreases with the increase of the concentration, and when the CD25 antigen polypeptide concentration is 10-15 μg / ml, the SBI value gradually increases with the increase of the concentration. This SBI binding curve shows that when the CD25 antigen polypeptide is at a lower concentration of 5 μg / ml (0.5 μg / well), the bottom of the 96-well microtiter plate is not covered, resulting in high non-specific reactions, so the SBI value at this time If it is too high, it is a false positive result; as the concentration of CD25 antigen polypeptide increases, the antigen gradually covers the entire bottom of the plate, its blocking effect appears, and the non-specific reaction gradually decreases, and the non-specific reaction is the lowest at 10 μg / ml. The specific binding between the antigen polypept...
Embodiment 2
[0040] kit preparation
[0041] 1 Reagents See Tab.2-9 for reagent preparation.
[0042]
[0043]
[0044] 2 operation
[0045] (1) Coating: Dilute the working antigen and reference antigen with coating solution to the working concentration, coat on the microtiter plate, and overnight at 4°C.
[0046] (2) Add plasma (primary antibody): wash the ELISA plate 3 times with washing buffer, dilute the plasma to an appropriate concentration with the analysis solution, generally 1:200-1:500, 100 μl per well, incubate at 25°C or room temperature 2~3h;
[0047] (3) Secondary antibody incubation: wash with washing buffer for 3 to 5 times, dilute the secondary antibody standard solution IgG with analytical solution, add 200 μl to each well, and incubate at 25°C / room temperature for 2 hours;
[0048] (4) Color development: wash with washing buffer 3 to 5 times, add 100 μl of substrate color development solution to each well, and keep at room temperature in the dark for 15 to 30 mi...
Embodiment 3
[0051] Detection of CD25 Auto IgG Antibody in Patients with Lung Cancer
[0052] 1 Sample collection: 501 plasma samples from tumor patients and healthy people were collected. The healthy group consisted of 227 cases, with an average age of 57.07±10.36 years, including 134 males and 92 females. The lung cancer group consisted of 274 cases, with an average age of 57.5±9.2 years, including 177 males and 97 females. The healthy group and the lung cancer group were matched in gender and age and were comparable (P>0.05)
[0053] 2 Detection results: From Tab.10-11, it can be known that the area under the ROC curve (AU) of the IgG antibody ROC curve (AU) of the CD25 antigen polypeptide in the plasma of lung cancer patients is 0.7, the sensitivity is 35%, and the specificity is 90%. The positive rate of IgG antibody binding to CD25 polypeptide antigen in plasma of patients with lung cancer was significantly higher than that of healthy group (Z=-7.48, P<0.001). The above data fully...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com