Method for preparing gemcitabine hydrochloride
A technology of gemcitabine hydrochloride and hydrochloride, which is applied in the pharmaceutical field and can solve the problems of high industrialization cost and low overall yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
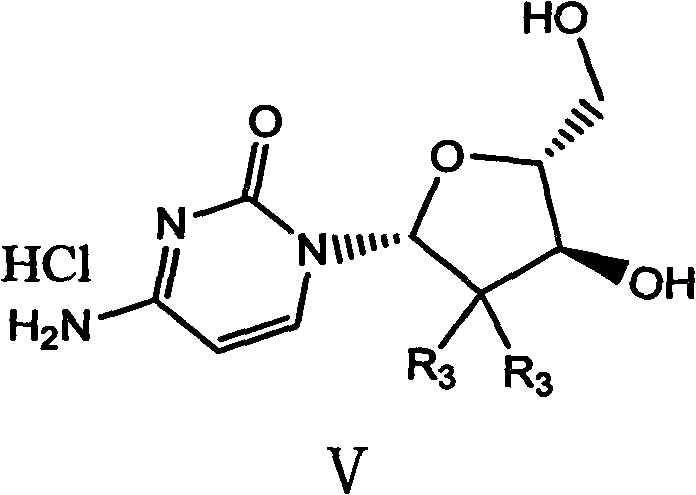
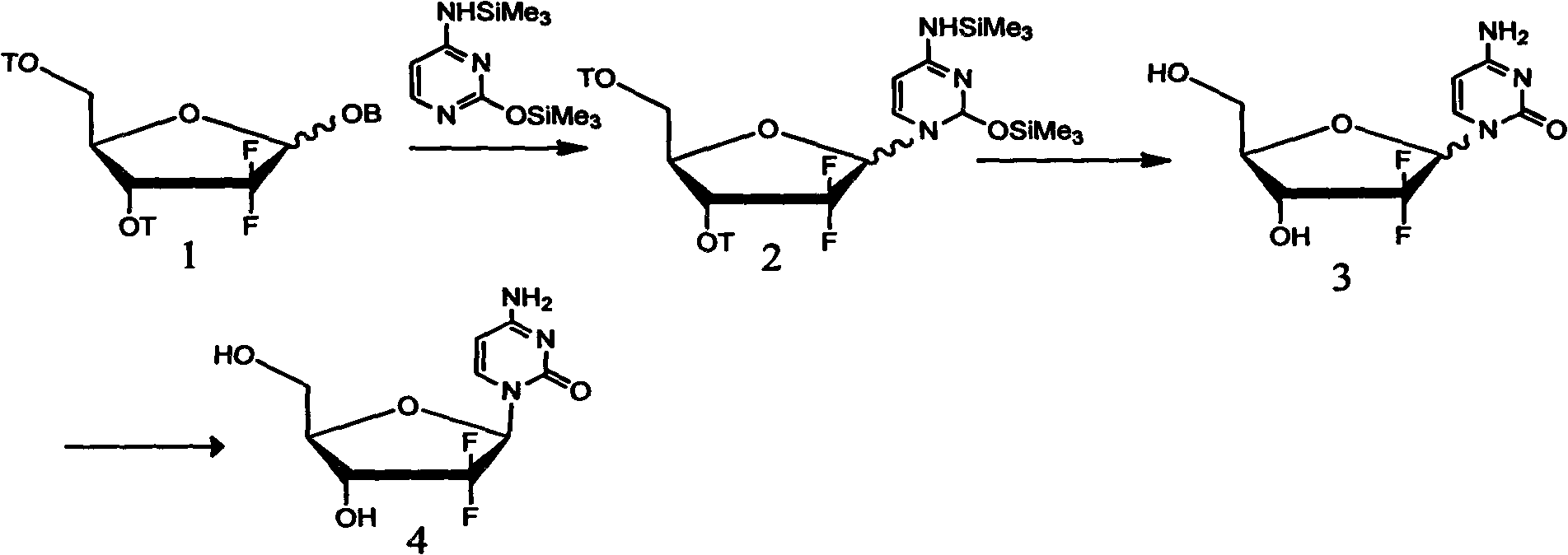
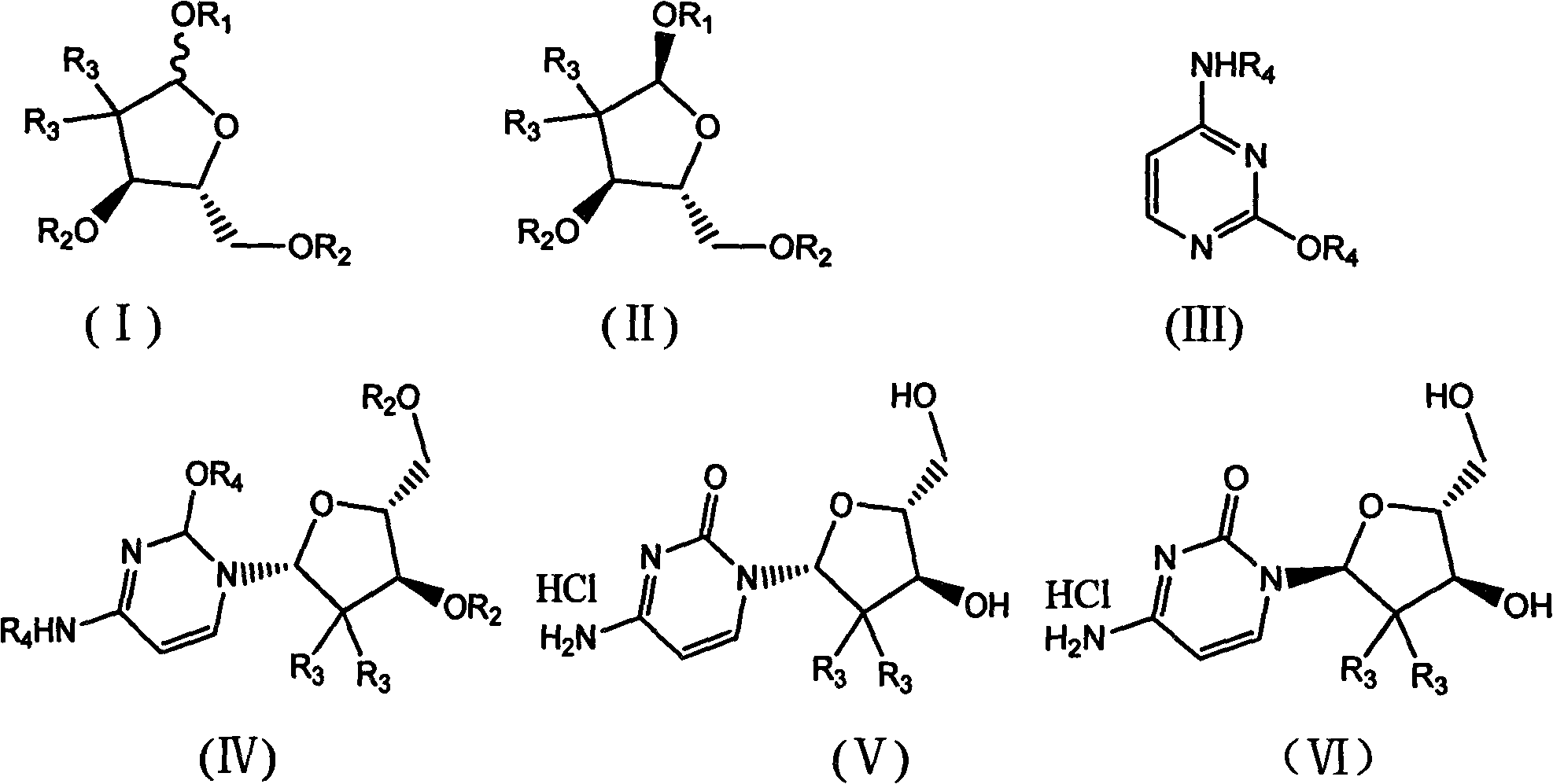
[0042] 1. Configuration transformation of 2-deoxy-2,2-difluoro-D-ribofuranosyl-3,5-ditoluoyl-1-methanesulfonate anomer mixture of formula i
[0043]
[0044] 1.64 g of triethylamine and 24.3 g of acetonitrile were added to the reaction flask and stirred. At 20°C-30°C, 1.56 g of methanesulfonic acid was added dropwise. After the dropwise addition, 6.25 g of 2-deoxy-2,2-difluoro-D-ribofuranosyl-3,5-ditoluoyl-1-mesylate i-anomer mixture (wherein the α-terminal The molar ratio of isomer to beta anomer is 1:1) was dissolved in 24 g of acetonitrile and charged into the reaction flask.
[0045] The temperature rises to room temperature, continues to heat up to reflux, and keeps warm for 20-24 hours. After the heat preservation is over, cool down to 20-30°C, and recover acetonitrile by vacuum distillation. After recovery, add 75g of water and 16g of dichloromethane, stir, separate layers, The phase was extracted twice with 10g×2 dichloromethane, the combined organic phases were w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com