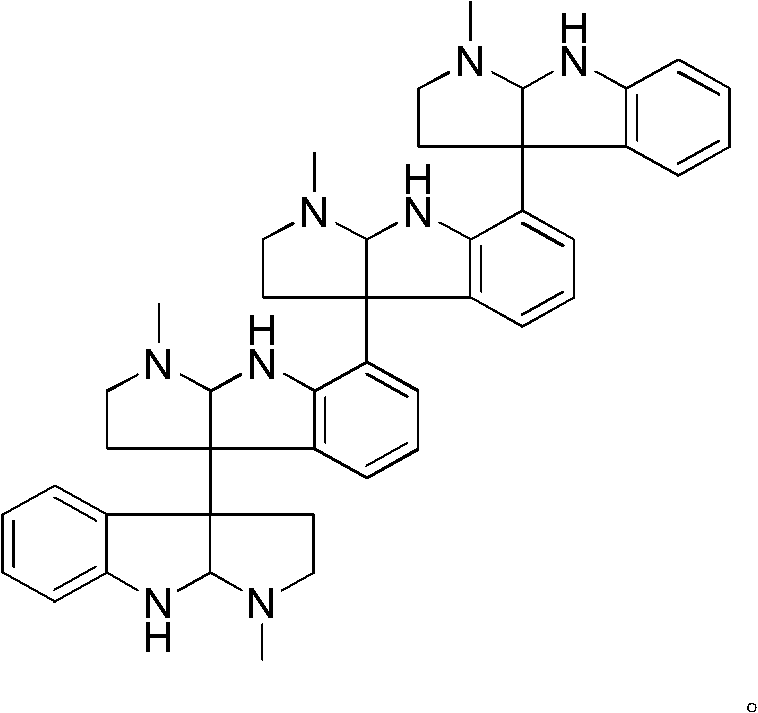
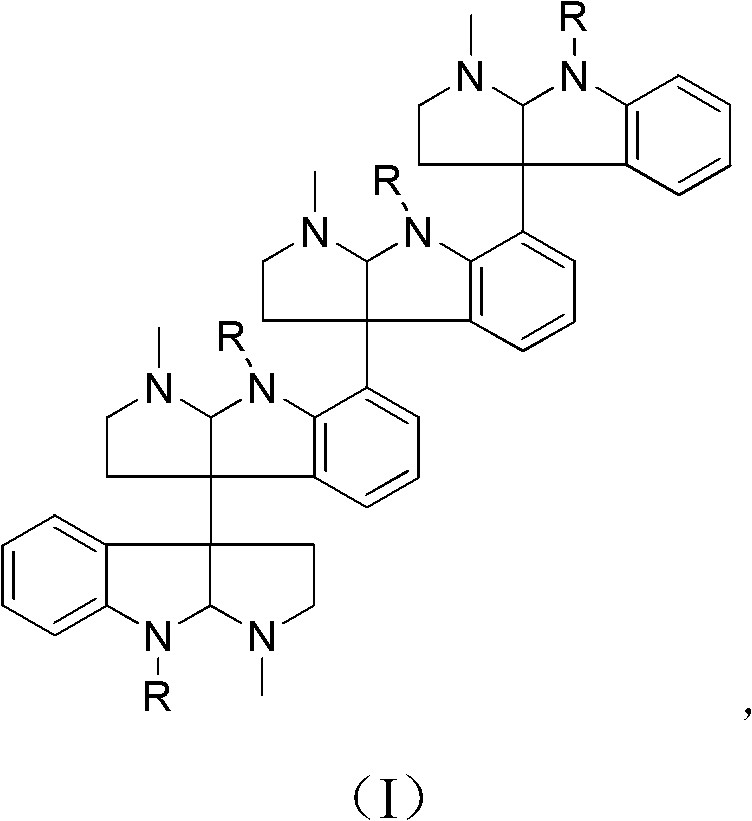
Indole alkaloid compound, its analog, its medicinal composition, and its use
A technology for medicinal salts and derivatives, which is applied in the field of preparation of drugs for the treatment or prevention of cancer, and can solve the problems of no reports on the medicinal activity of compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] Compound preparation:
[0085] After air-drying, 9 kg of plant hair nine knots (Psychotria pilifera) were heated (70° C.) with 90% ethanol to reflux and extracted 3 times, the solvent was recovered, concentrated to a small volume, 1% HCl was added to adjust the pH value to 2, and extracted with ethyl acetate for 3 The aqueous layer was adjusted to pH 9 with 10% ammonia water, extracted three times with ethyl acetate, and the ethyl acetate layer was concentrated and weighed to obtain 98 g. After the ethyl acetate part was mixed with an appropriate amount of silica gel, silica gel column chromatography (400g, 200-300 mesh, Qingdao Ocean Chemical Factory) was divided into 6 sections (Frs 1-6), and chloroform-methanol gradient elution (1: 0, 40:1, 20:1, 10:1, 6:1, 0:1). Fr5 (11.5 g) was eluted with methanol-water (2:3, 1:1, 3:1, 4:1, 1:0) on a RP-18 reverse phase column to obtain three fractions A, B, and C. Fraction B was eluted through a forward silica gel column with c...
Embodiment 2
[0093] Compound preparation:
[0094] After air-drying, 1 kg of plant hairy nine knots (Psychotria pilifera) were cold-soaked and extracted 5 times with 90% ethanol, recovered the solvent, concentrated to a small volume, added 1% HCl to adjust the pH value to 2, extracted 3-5 times with acid water, combined acid The water extract was passed through 732 strong acid ion exchange resin, eluted with water for 4 times the column volume, the water eluate was discarded, and then washed with 4 times the column volume of the mixed solution of ethanol:water:ammonia water (70:25:5), The eluate was concentrated and weighed to obtain 16 g. Carry out silica gel column chromatography (400g, 200-300 mesh, Qingdao Ocean Chemical Factory) is divided into 6 sections (Frs 1-6), use chloroform-methanol gradient elution (1:0, 40:1, 20:1,10 :1, 6:1, 0:1). Fr5 (11.5 g) was eluted with methanol-water (2:3, 1:1, 3:1, 4:1, 1:0) on a RP-18 reverse phase column to obtain three fractions A, B, and C. Fr...
Embodiment 3
[0096] Compound preparation:
[0097] After air-drying, 1kg of Psychotria pilifera was extracted by heating and refluxing with methanol for 5 times, the solvent was recovered, concentrated to a small volume, the pH value was adjusted to 2 by adding 1% HCl, extracted 3-5 times with acid water, combined with acid water extraction The product was passed through LSI-296 weak acid ion exchange resin, eluted with water for 4 times of the column volume, the water eluent was discarded, and then washed with 4 times of the column volume of the mixed solution of ethanol:water:ammonia water (70:25:5), The eluate was concentrated and weighed to obtain 17 g. Carry out silica gel column chromatography (400g, 200-300 mesh, Qingdao Ocean Chemical Factory) is divided into 6 sections (Frs 1-6), use chloroform-methanol gradient elution (1:0, 40:1, 20:1,10 :1, 6:1, 0:1). Fr5 (11.5 g) was eluted with methanol-water (2:3, 1:1, 3:1, 4:1, 1:0) on a RP-18 reverse phase column to obtain three fraction...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com