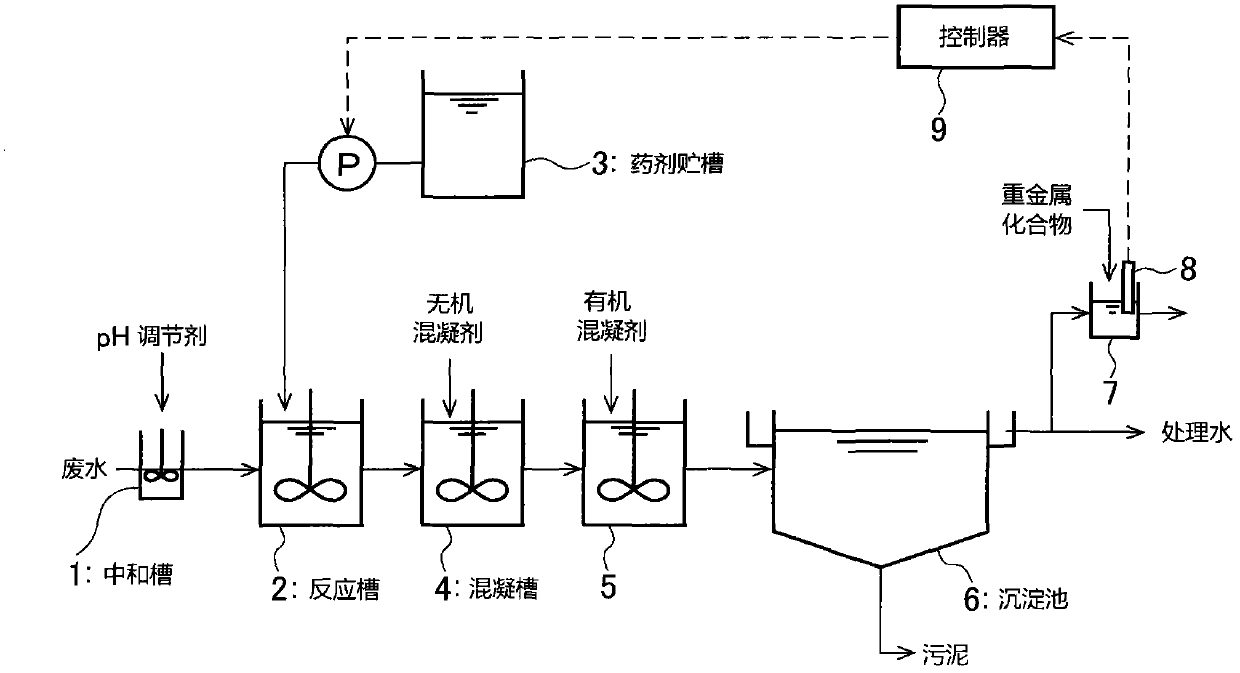
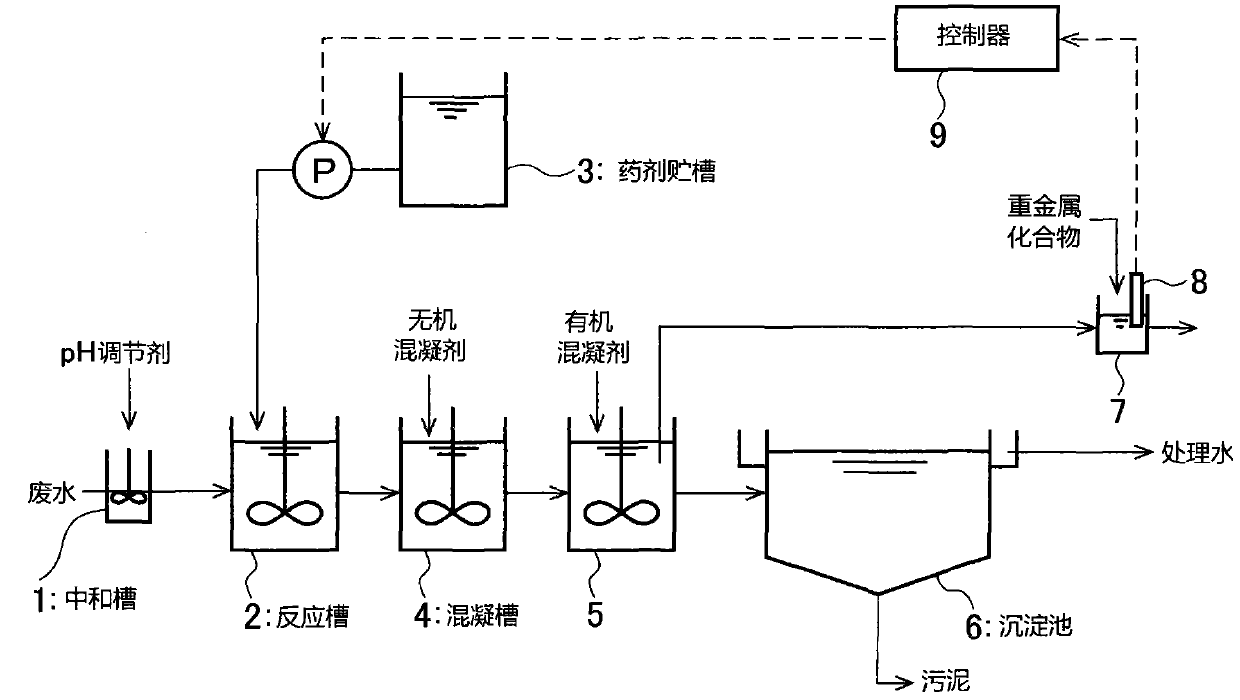
Adding control method of heavy metal trapping agent
A heavy metal trapping agent and control method technology, applied in chemical instruments and methods, material analysis by observing the impact on chemical indicators, analysis by making materials undergo chemical reactions, etc., can solve the problem of reducing water quality and increasing disulfide Substituting carbamic acid heavy metal trapping agent cost and other issues, to achieve the effect of low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
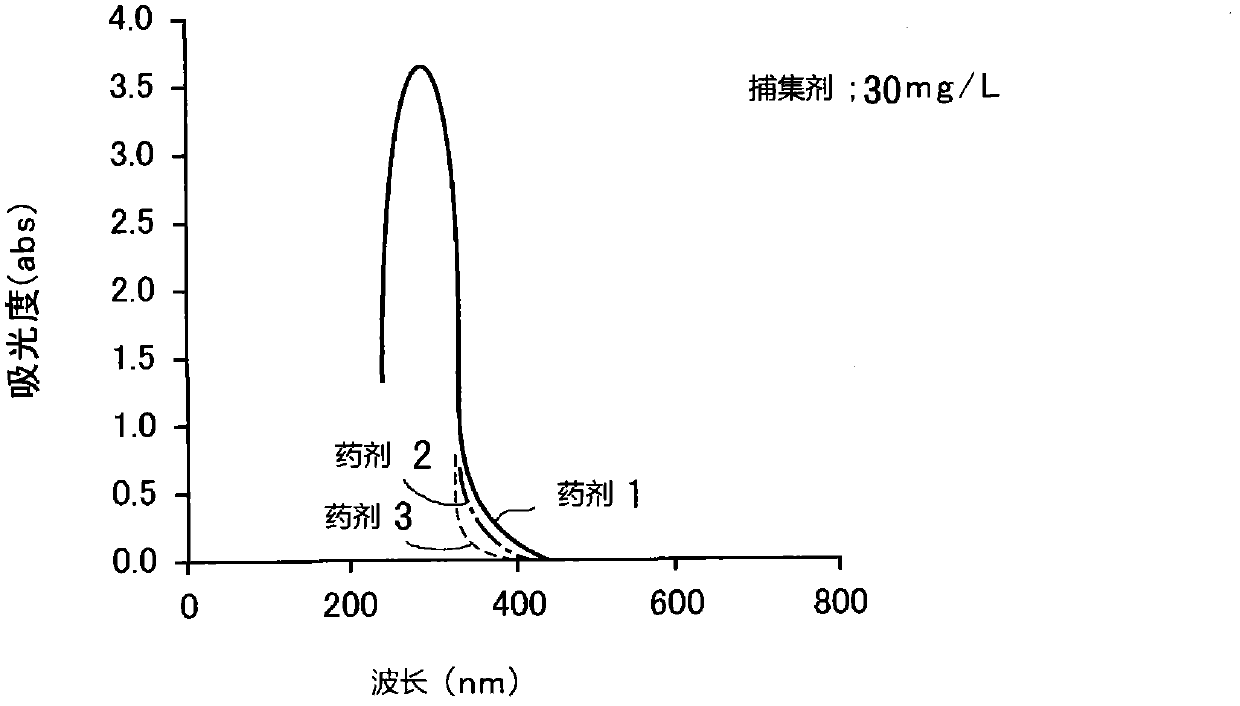
[0069] Absorbance spectra were measured for four kinds of agents 1 to 4 in Table 1 as dithiocarbamic acid heavy metal trapping agents and are shown in image 3 middle. As shown in the figure, any collector has a strong absorption band at a wavelength of 200-350 nm and an absorption peak around a wavelength of about 240 nm. Also, the absorbance at wavelengths above 400 nm is very small.
[0070] Table 1
[0071]
experiment example 2
[0073] In water dissolved with 30 mg / L of agent 3 as a trapping agent, as Fe 2+ Add 10mg / L FeSO 4 , and measure the absorbance spectrum. The results are shown in Table 4. exist Figure 4 also shows that no FeSO 4 time spectrum.
[0074] Such as Figure 4 As shown, by adding Fe 2+ , and it was confirmed that strong absorption peaked at a wavelength of 420 nm.
[0075] Therefore, the determination of the change in FeSO relative to a 30 mg / L aqueous solution of agent 4 The absorbance at 470nm wavelength when the added amount is shown in Figure 5 middle. Such as Figure 5 As shown, in Fe 2+ The absorbance reached the limit when the amount added was 10 mg / L or more, and it was confirmed that Fe 2+ The equivalent reaction amount with 30 mg / L of drug 3 is 10 mg / L.
[0076] Therefore, the concentration of the drug 3 is set in the range of 3 to 30 mg / L, and the amount of Fe that is more than the equivalent reaction amount is measured. 2+ Add 10mg / L FeSO 4 Absorbance at ...
experiment example 3、4
[0078] In order to confirm the removal of Fe 2+The color development of heavy metal ions other than , measured in the aqueous solution of 30mg / L agent 3 as Cu 2+ Add 20mg / L of CuSO 4 The spectrum at the time is shown in Figure 7 Medium (experimental example 3). In addition, in the aqueous solution of 30 mg / L drug 3, Fe 3+ Add 10mg / L of Fe 2 (SO 4 ) 3 The spectrum at the time is shown in Figure 8 Middle (experimental example 4).
[0079] As a result, it was confirmed that the Cu 2+ , Fe 3+ The case also reacts with agent 3 at about 460nm (Cu 2+ case) or 360nm (Fe 3+ case) produces a color development with a peak. And confirmed that when adding Al 3+ , Ca 2+ when, such as Figure 8 No color developed as shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com