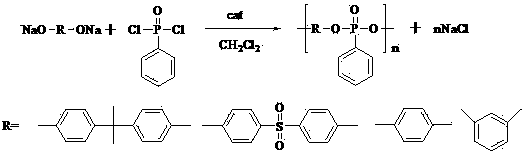
Method for synthesizing polymerized phenyl phosphonate by performing solid-liquid two-phase reaction
A phenylphosphonate, polymerized technology, applied in the field of solid-liquid two-phase reaction synthesis of polymerized phenylphosphonates, can solve the problems of high volatility, decreased physical properties, poor heat resistance, etc., and achieves a high degree of polymerization. , the reaction steps are simple, the thermal stability is good
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Dissolve 170g of NaOH in 4L of water to prepare NaOH solution, add 456g of bisphenol A into the reaction vessel, slowly pour all the NaOH solution into the reaction vessel, under the protection of nitrogen, stir at room temperature until completely dissolved, and form a salt . The water was distilled off under reduced pressure, and after vacuum drying, 530 g of bisphenol A disodium salt was obtained as a white solid.
Embodiment 2
[0030] Dissolve 170 g of NaOH in 4L of water to prepare a NaOH solution, add 500 g of bisphenol S into the reaction vessel, slowly pour all the NaOH solution into the reaction vessel, and stir at room temperature until completely dissolved under nitrogen protection to form a Salt. Water was distilled off under reduced pressure, and 560 g of bisphenol S disodium salt was obtained after vacuum drying.
Embodiment 3
[0032] Put 500g of bisphenol A disodium salt into the reaction vessel, weigh 70g of tetrahexylammonium chloride and put it into the reaction vessel, weigh 360g of phenylphosphonic acid dichloride and dissolve it in 12L of dichloromethane. The dichloromethane solution of acid dichloride was poured into the reaction vessel, and the temperature was raised to reflux for 2 h. The reaction mixture was washed with water for 3-4 times, the organic phase was separated, and the organic phase was concentrated to about 1.2 L, then 25 L of petroleum ether was poured into the organic phase, left to stand, the liquid was decanted, and the product was precipitated at the bottom. The product at the bottom was dried at 70°C, agglomerated into a solid after cooling, and pulverized.
[0033] The product was a white waxy solid with a mass of 601 g and a yield of 93.2%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com