Combination therapy using a beta 3 adrenergic receptor agonist and an antimuscarinic agent
A muscarinic, agonist technology, used in drug combinations, animal repellents, botanical equipment and methods, etc., can solve problems such as insufficient response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-3
[0425] Materials and methods
[0426] The following materials and methods were used in Examples 1-3. Male mature Sprague-Dawley rats were used. Use CO 2 After gas euthanasia, the entire bladder was removed. A longitudinal strip (approximately 6 mm × 3 mm) of the outer triangular portion of the detrusor muscle was prepared. Place each strip in an atmosphere containing oxygen (95% O2 + 5% CO 2) in a heated (37°C) organ bath (25 mL) of Krebs solution. One end of the strip was fixed to an organ bath and the other end was connected to an isometric transducer (AD Instruments) under a resting tension of 10 mN. The responses of the prepared samples were recorded at a sampling frequency of 10 Hz using a multi-channel data acquisition system (PowerLab, AD Instruments), and measured using analysis software (Chart, AD Instruments). After an equilibration time of at least 60 min, an electric field stimulus (EFS) at 60 Hz; duration, 0.3 ms; 3 s; 90 V was applied to each tissue strip...
Embodiment 2
[0431] β 3 Combination therapy of the AR agonist CL316243 with an antimuscarinic agent selected from tolterodine, oxybutynin and darfinacin
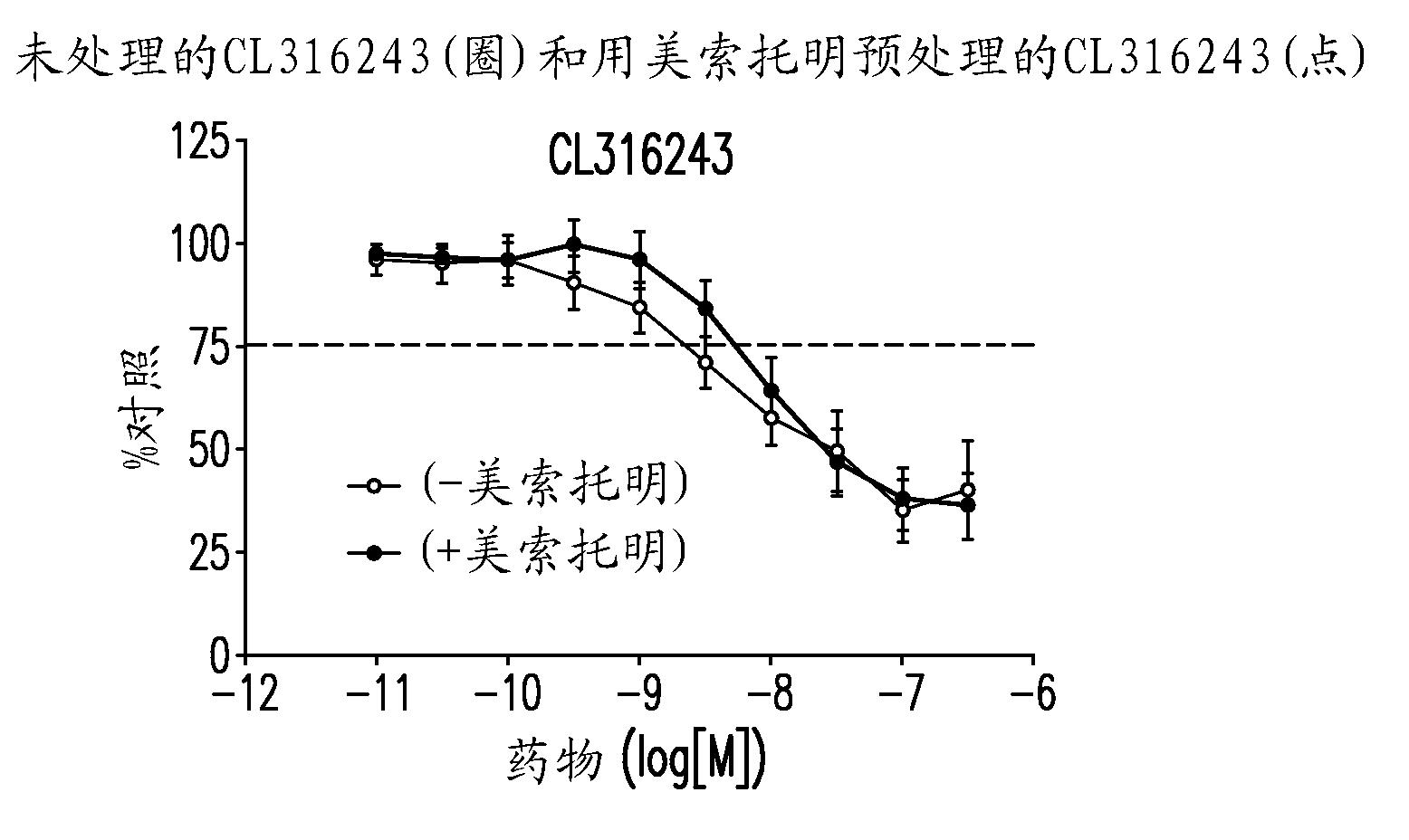
[0432] When administered alone, CL316243, tolterodine, oxybutynin, and darfenacin all inhibited EFS-induced isolated detrusor contractions. Table 5 shows the concentration of each compound that induced 25% inhibition. These values are used in the isobologram analysis below.
[0433] Table 5. Inhibition of Detrusor Contraction by CL316243, Tolterodine, Oxybutynin, and Darfinacine
[0434]
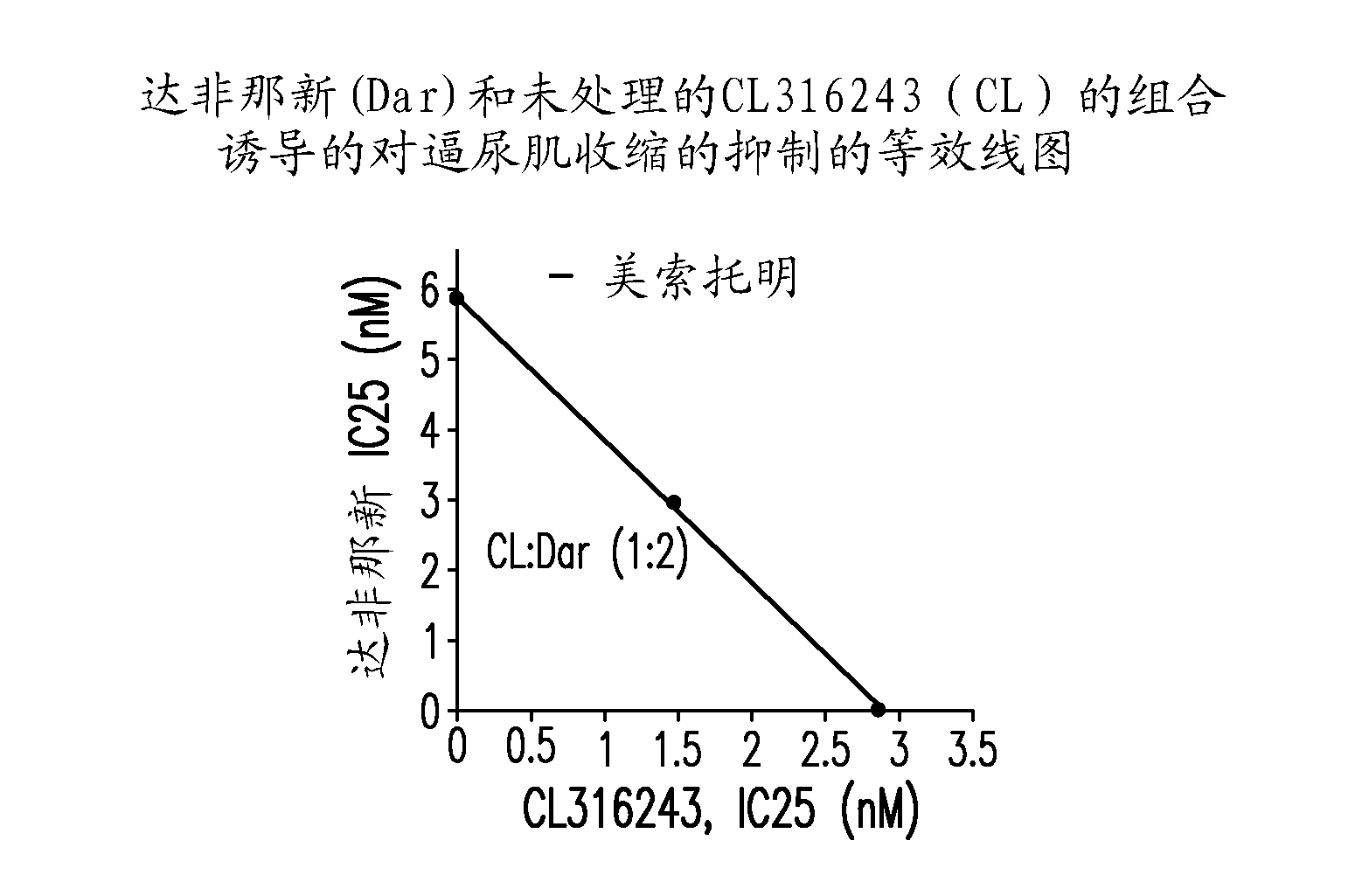
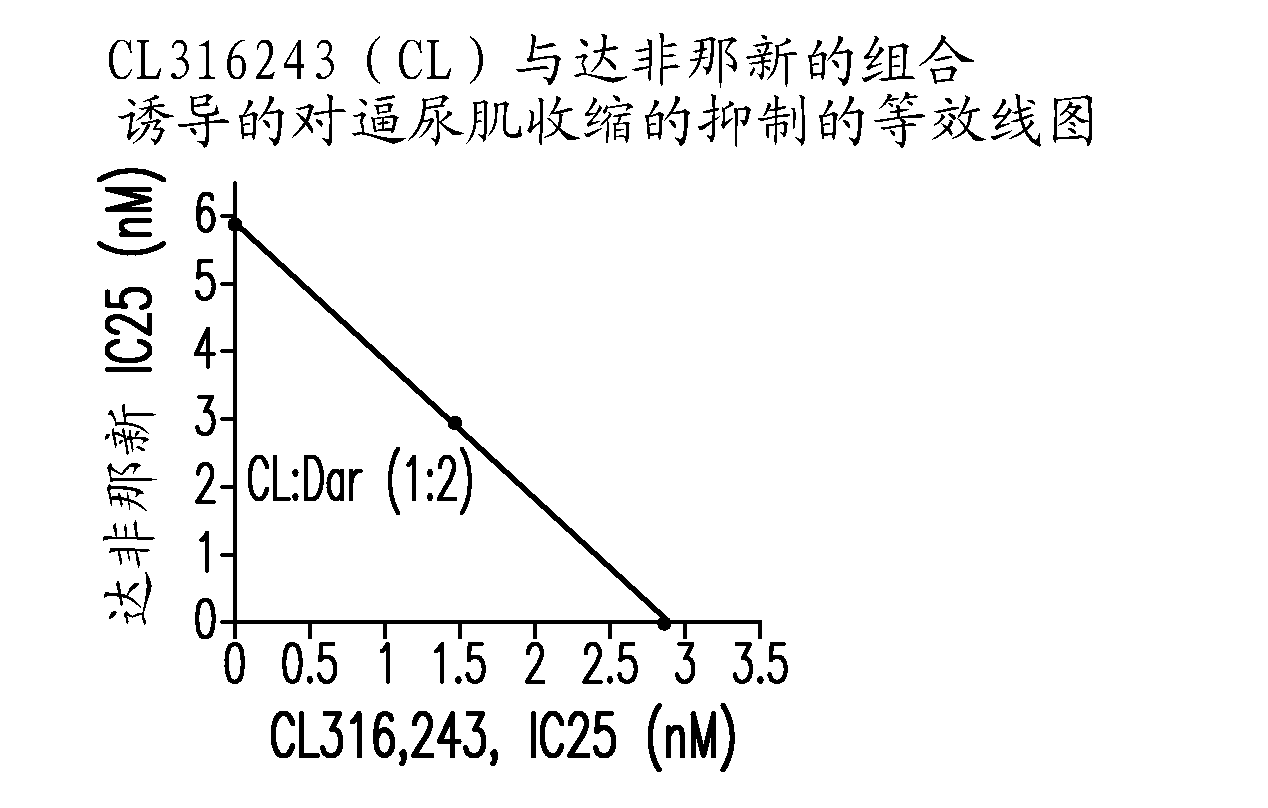
[0435] In the combination therapy, CL316243 was administered with tolterodine, oxybutynin and darfinacin at a fixed weight ratio, and the results of the isobologram analysis are shown in Figure 2. Fig. 2 shows CL316243 and tolterodine (1:2, Figure 2A ) or oxybutynin (1:10, Figure 2B ) show a synergistic effect. On the other hand, CL316243 and darfenazine (1:2, Figure 2C ) shows simple addition (ie, no synergy).
[0436] While not ...
Embodiment 3
[0441] β 3 - Combination of AR agonist compound 12 with tolterodine or darfinacine
[0442] In this example, different β 3 -AR agonists to study beta 3 -Synergistic effect of combination therapy of an AR agonist and an antimuscarinic agent.
[0443] β as described in Table 3 above 3 IC of -AR agonist compound 12 inhibits EFS-induced isolated detrusor contraction 25 The value is 275 nM. Thus, compound 12 was more potent than CL316243 in inhibiting EFS-induced contraction of rat bladder strips (IC 25 2.86 nM, see Table 5) was 100-fold lower. This is consistent with the effect of compound 12 on rat β 3 The low potency activity of -AR is consistent.
[0444] In the combination study, compound 12 was administered with tolterodine or darfenacin at a fixed weight ratio of 50:1. The results of isobologram analysis are shown in Fig.3. Figure 3 shows that compound 12 has a M of about 1 2 / M 3 The 50:1 combination of tolterodine ( Figure 3A ) have a synergistic effect...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com