Synergistic antiviral composition and use thereof
A composition and virus technology, applied in the field of immunology, can solve problems such as inability to achieve therapeutic treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
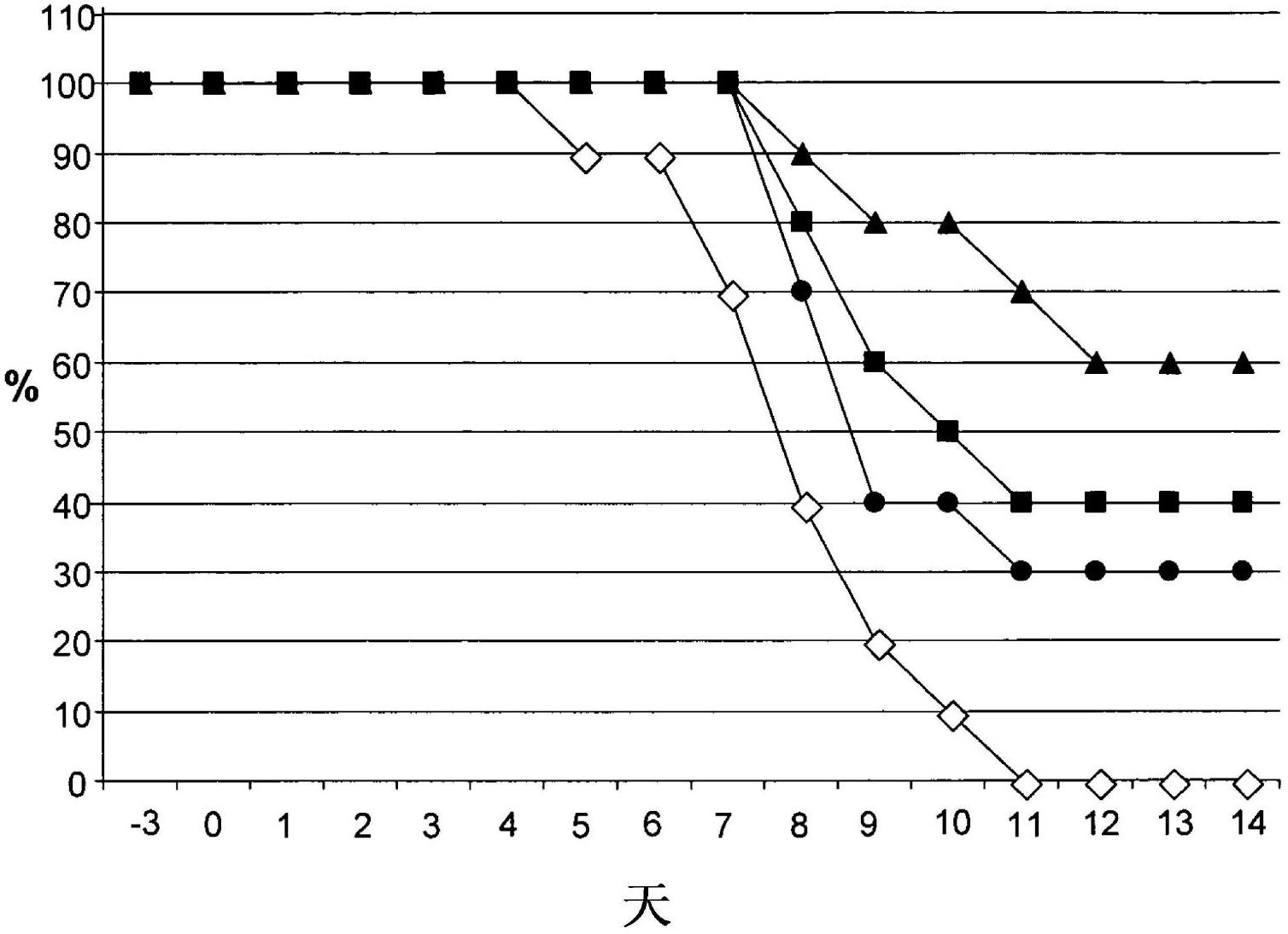
[0052] Example 1: iota-carrageenan and TAMIFLU in mice treated 48 hours after infection synergistic therapeutic effect.
[0053] Lethal dose (10xLD) was utilized by intranasal instillation (i.n.) without anesthesia 50 ) of influenza A / PR / 8 / 34 virus infected 40C57BI6 mice. Following nasal infection, the virus spread into the respiratory tract and eventually caused fatal pneumonia in mice. The model simulates natural influenza infection in humans via the respiratory tract, which sometimes leads to severe illness.
[0054] The mice were divided into four groups with 10 mice in each group. Forty-eight hours after infection, mice were treated twice daily with intranasal instillation without anesthesia, where the first group received placebo treatment (25 μl of 0.5% NaCl in water per nostril) as a negative control, and the second group received only Each nostril was treated with 25 μl of an aqueous solution of iota-carrageenan polymer at a concentration of 1200 μg / ml. Groups...
Embodiment 2
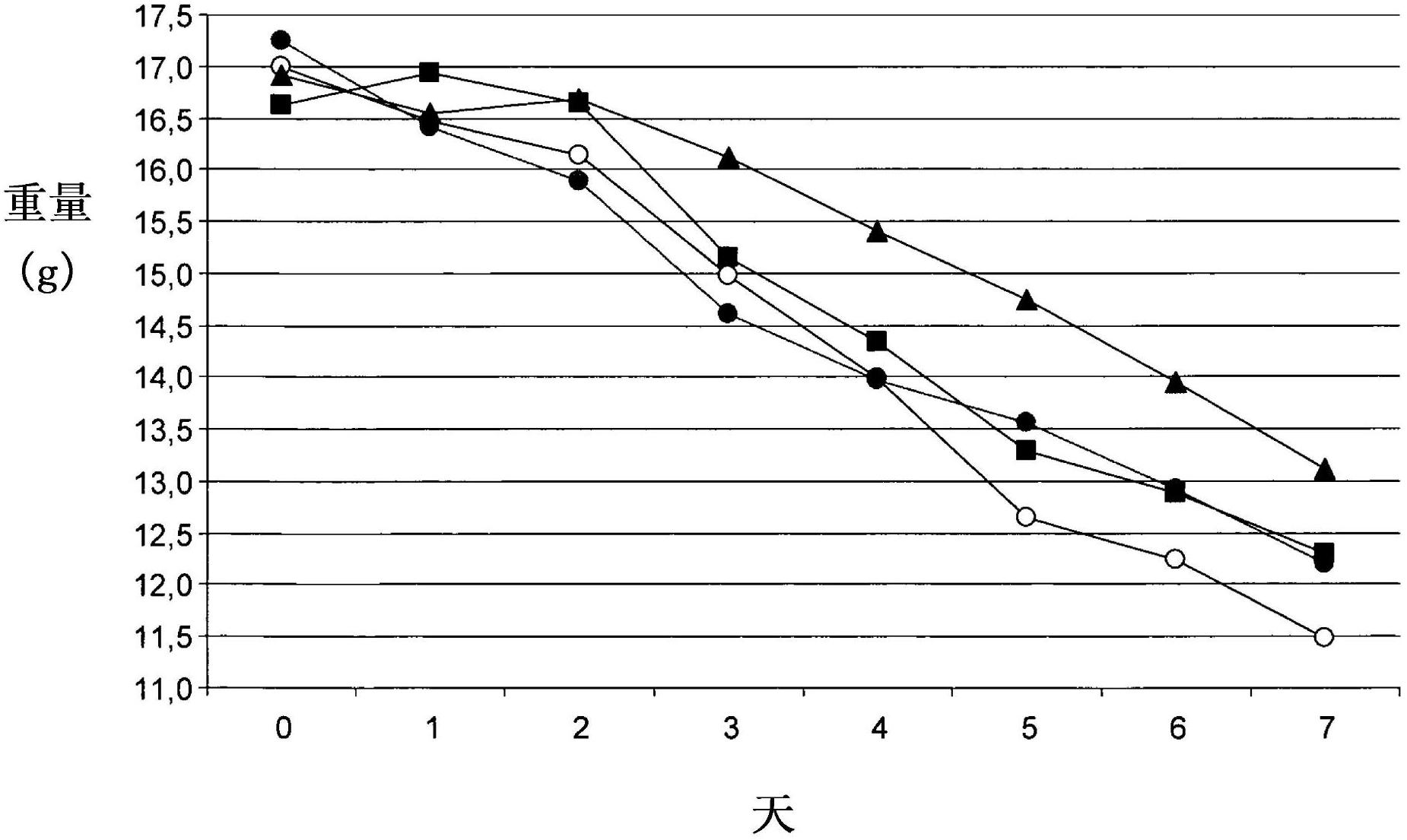
[0057] Example 2: iota-carrageenan and TAMIFLU in mice treated 24 hours after infection synergistic therapeutic effect.
[0058] Due to TAMIFLU (oseltamivir phosphate) is known to be most effective when treatment is initiated within 24 hours of the first symptoms of infection, so it was analyzed whether combination treatment with iota-carrageenan could improve TAMIFLU of therapeutic efficacy. Due to the use of TAMIFLU Treatment already provided a high degree of protection against lethal outbreaks of the disease, so average animal weight was used as a surrogate parameter to assess animal health. Animal weights were compared using a t-test. The experiments were carried out as described in Example 1, with the only difference that the treatment was started up to 24 hours after infection.
[0059] like figure 2 As shown, all animals suffered from weight loss due to lethal influenza virus infection. While using TAMIFLU (black circles) or iota-carrageenan (black squar...
Embodiment 3
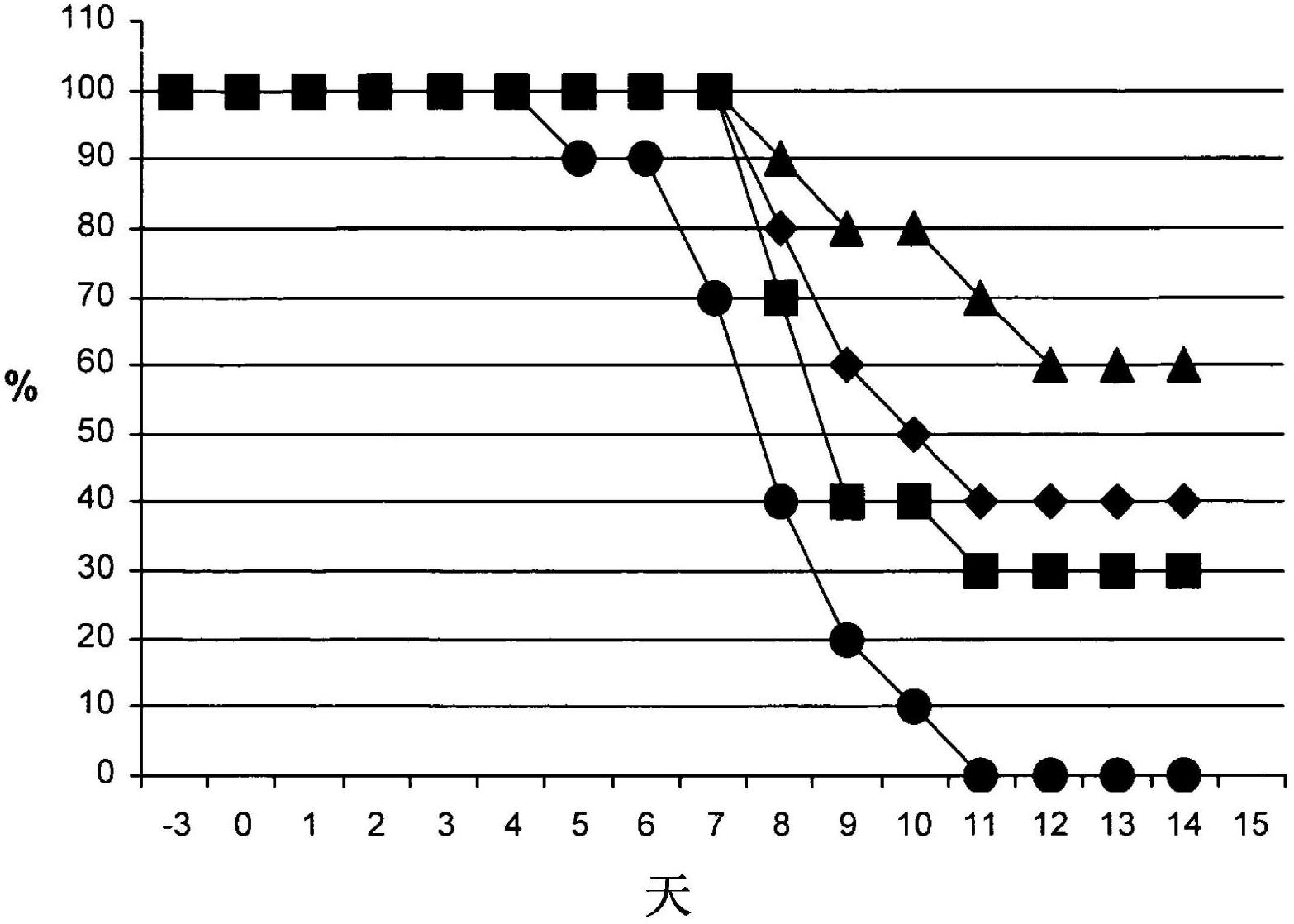
[0060] Example 3: Synergistic therapeutic effect of iota-carrageenan and oseltamivir in mice treated 48 hours after infection.
[0061]Infection of mice was accomplished as described in Example 1. The mice were divided into four groups with 10 mice in each group. Forty-eight hours after infection, mice were treated twice daily with intranasal instillation without anesthesia, where the first group received placebo treatment (25 μl of 0.5% NaCl in water per nostril) as a negative control, and the second group received doses of Oral treatment with oseltamivir at 5 mg / kg / day, the third group was treated with 25 μl of an aqueous solution of iota-carrageenan polymer at a concentration of 1200 μg / ml in each nostril, and the fourth group was treated with iota-carrageenan twice a day. - combination therapy of carrageenan and oseltamivir, wherein oseltamivir is administered orally at a dose of 5 mg / kg / day, and the total amount of intranasally administered iota-carrageenan is 100 μl, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com