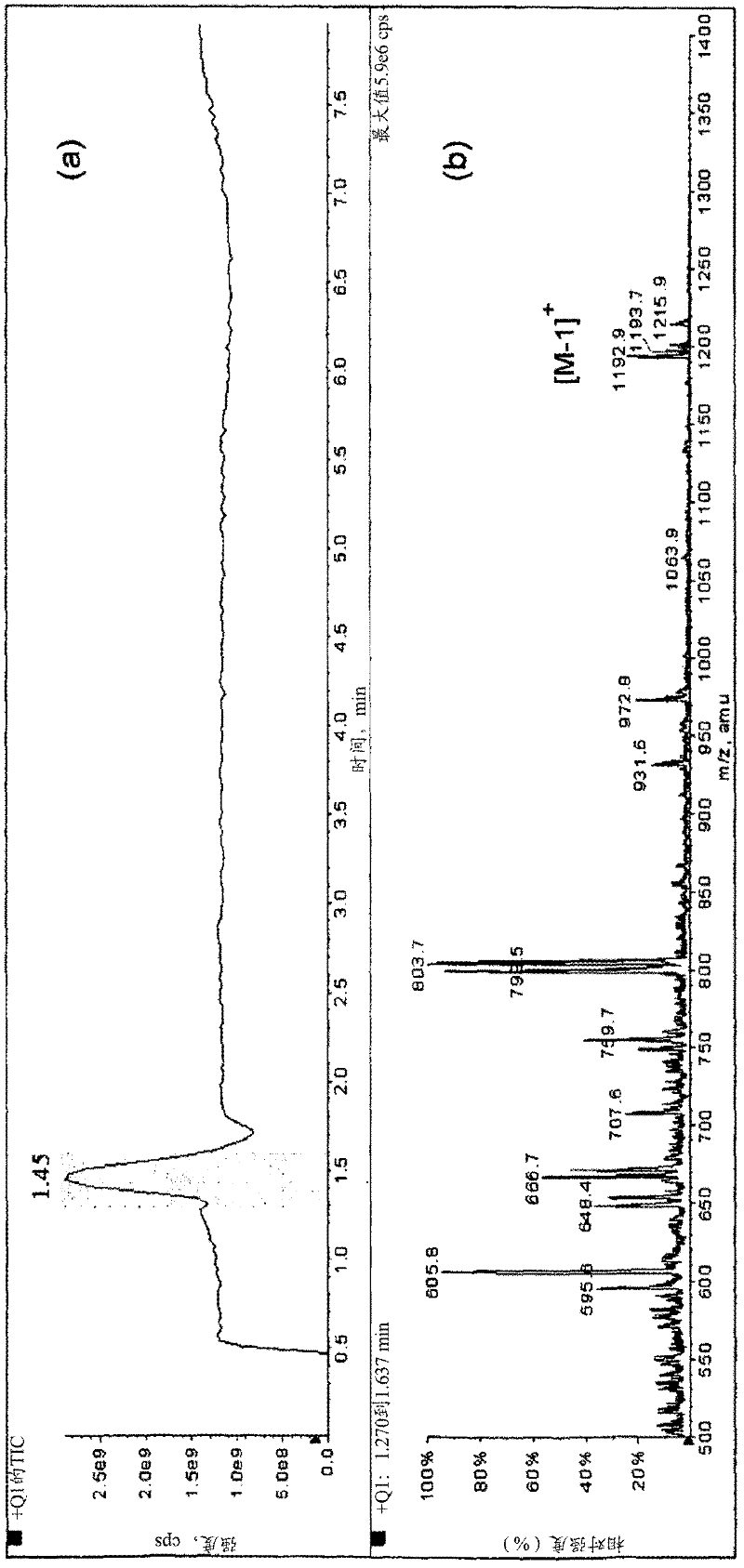
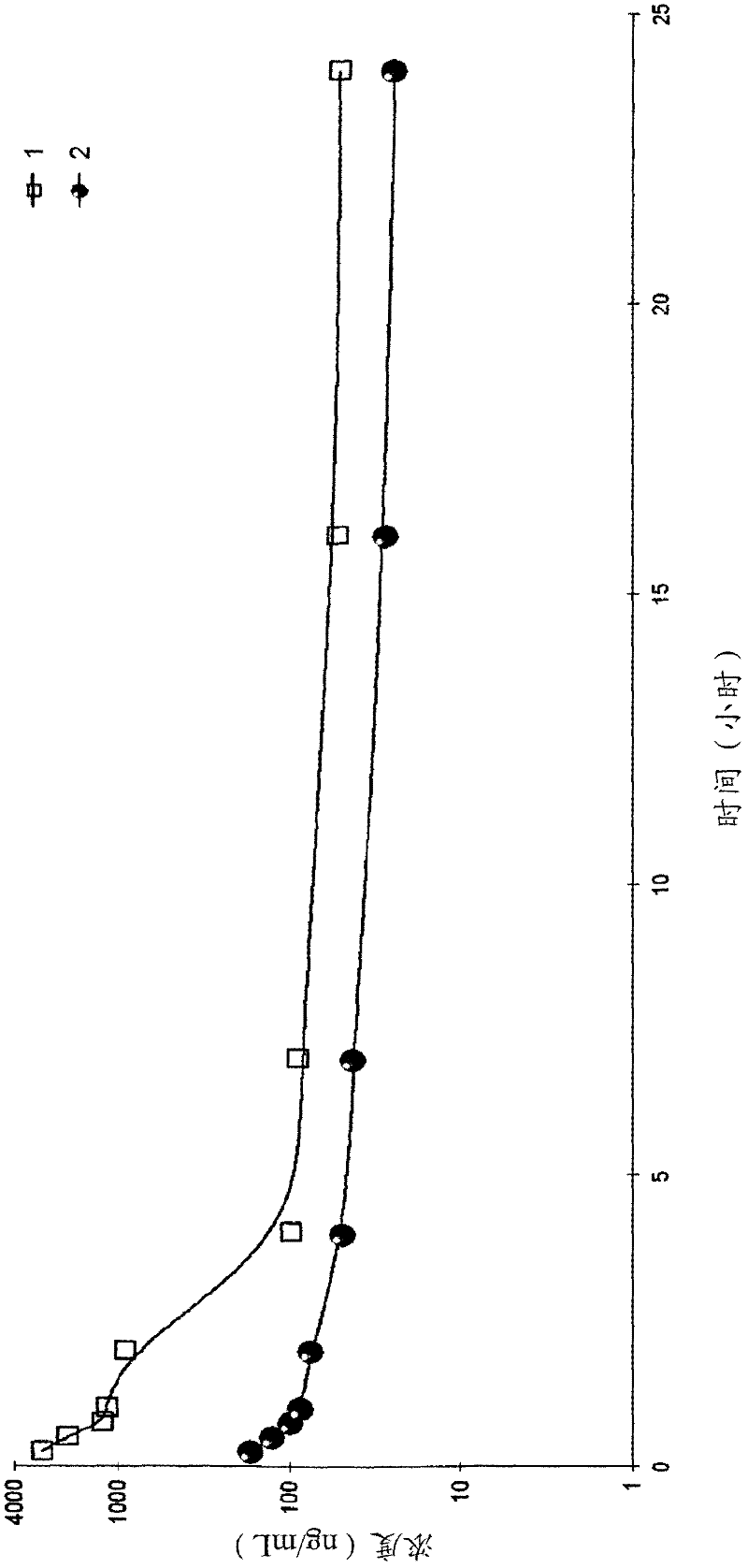
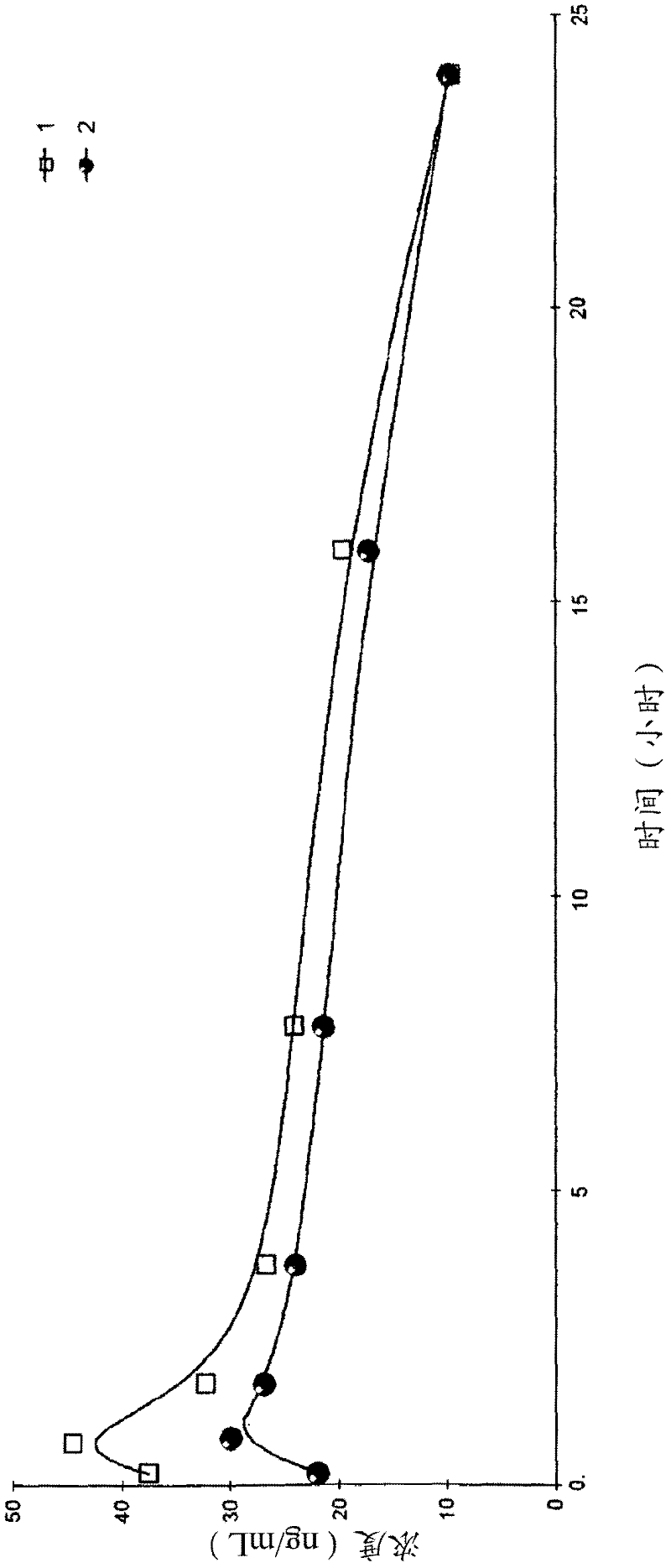
Pure PEG-lipid conjugates
A technology of conjugates and lipids, applied in the field of PEG-lipid conjugates of PEG chains, which can solve problems such as expensive
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0195] Embodiment 1.3-oleoyl-1, the synthesis of 2-two (methoxyhexaethylene glycol) glycerol
[0196] Part 1A: 3-Benzyl-1,2-di(methoxyhexaethylene glycol)glycerol
[0197] (±)-3-Benzyloxy-1,2-propanediol (1.2 g, 6 mmol), NaH (0.96 g, 40 mmol) and dry THF (150 mL) were added into a three-necked flask. A dry THF solution (50 mL) of monomethoxyhexaethylene glycol tosylate (5.4 g, 12 mmol) was then added dropwise to the mixture at room temperature. The mixture was refluxed for 24 hours and cooled to room temperature. Ice-cold methanol was added to the reaction mixture to quench excess NaH. Evaporate the solvent and wash with 5% HCl (w / v) and CH 2 Cl 2 The crude product was extracted. The solvent was evaporated and further purified by gel permeation chromatography to give 85% colorless liquid.
[0198] Part 1B: 3-Hydroxy-1,2-bis(methoxyhexaethylene glycol)glycerol
[0199] 5 drops of acetic acid and 0.6 g of palladium black were added to a solution of 5 g of 3-benzyl-1,2-di(...
Embodiment 21
[0204] Example 2.1,2-dioleoyl-racemic-3-monomethoxy dodecaethylene glycol (mPEG-12)-glycerol
[0205] The general steps used for this synthesis are shown schematically in Reaction Scheme 8.
[0206]
[0207] Reaction Scheme 8: Synthesis of 1,2-isopropylidene-glycerol-3-monomethoxydodecyl glycol ester
[0208] 1 mole of hexaethylene glycol is mixed with 0.15 mole of pyridine and heated to 45-50°C, and 0.1 mole of trityl chloride is added. The reaction was carried out overnight (approximately 16 hours) with continuous stirring, then cooled to room temperature and extracted with toluene. The extract was washed with water, then extracted with hexane, and washed with MgSO 4 dry. The solvent was removed in vacuo to give trityl-hexaethylene glycol as a pale yellow oil (70-85% yield).
[0209] 0.1 mole of trityl-hexaethylene glycol and 0.101 mole of p-toluenesulfonyl chloride were mixed in 100 mL of dichloromethane. The homogeneous mixture was cooled to 0 °C in a dry ice aceto...
Embodiment 31
[0220] Example 3.1,2-Dioleoyl-racemic-3-monomethoxydodecaethylene glycol (mPEG-12) glycerol
[0221] The general steps used for this synthesis are illustrated in the following scheme (Scheme 9):
[0222]
[0223] Reaction Scheme 9: Synthesis of 1,3-dioleoyl-2-glyceride
[0224] 0.033 molar dihydroxyacetone was stirred continuously in 150 mL of chloroform under nitrogen. 0.06 mol of oleoyl chloride was dissolved in 150 mL of chloroform and added to this heterogeneous mixture of dihydroxyacetone, followed by 10 mL of anhydrous pyridine. The reaction was carried out at room temperature for 30 minutes with continuous stirring. The mixture became homogeneous and the reaction was complete when no oleoyl chloride was detectable in the mixture. The bulk solvent was removed under vacuum. The residue was washed with water, then extracted with ethyl acetate. The aqueous phase was repeatedly extracted with ethyl acetate, the combined organic layers were washed again with water, dr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com