New application of tetrandrine in fungal bio-film resistance
A technology of tetrandrine and biofilm, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
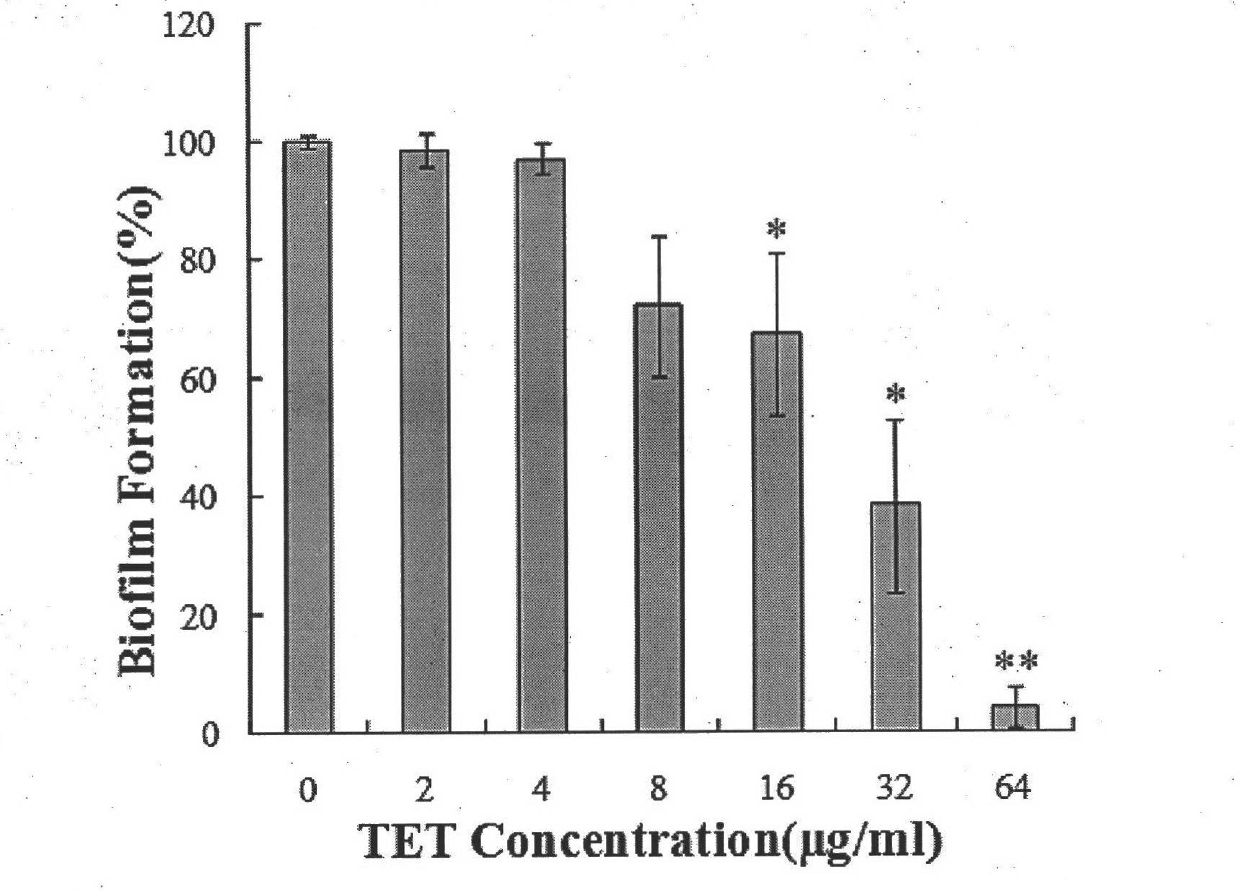
[0014] Example 1: The effect of Tetrandrine alone on the biofilm of different Candida albicans strains.
[0015] Materials and methods
[0016] 1. Test drug
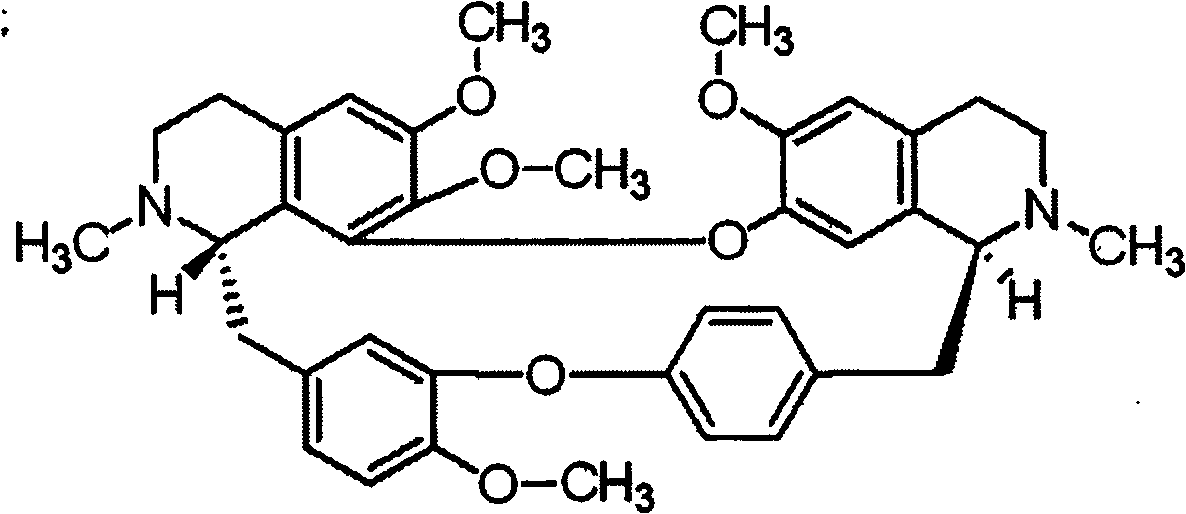
[0017] Tetrandrine: purchased from Sigma Company.
[0018] Fluconazole: purchased from Sigma Company.
[0019] Amphotericin B: purchased from Sangon Bioengineering Co., Ltd.
[0020] XTT: purchased from Sigma Corporation.
[0021] Menadione (menadione): purchased from Sigma Company.
[0022] Dimethyl sulfoxide (DMSO): China Pharmaceutical (Group) Shanghai Chemical Reagent Company, redistilled before use.
[0023] Acetone: purchased from Sigma Company.
[0024] Tetrandrine was made into 6.4 mg / ml and 12.8 mg / ml solutions with dimethyl sulfoxide, amphotericin B was made into 4 mg / ml solution with dimethyl sulfoxide, and fluconazole was made into 102.4 mg with dimethyl sulfoxide / ml solution, the test drug was stored at -20°C. Before the experiment, the drug stock solution was taken out and melted in a 35°C incubat...
Embodiment 2
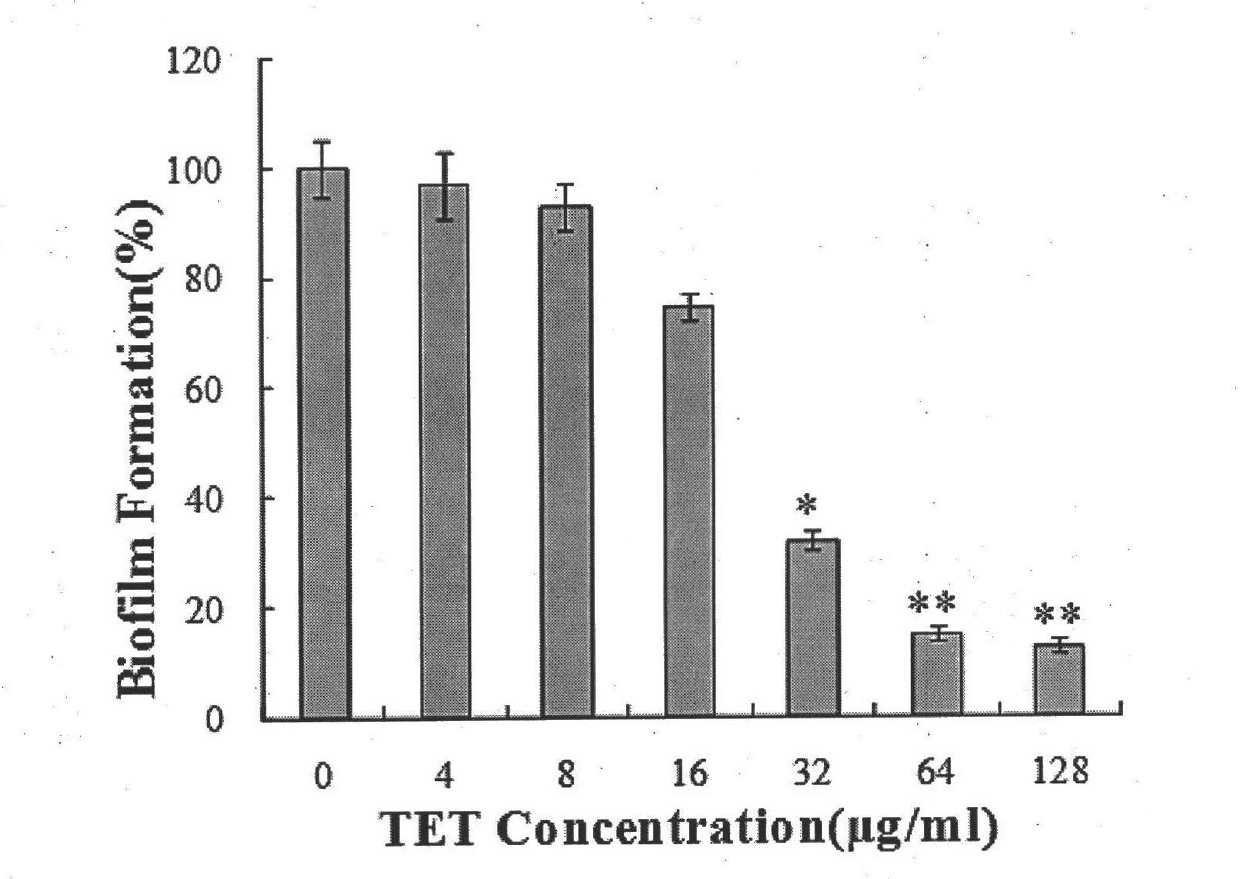
[0056] Embodiment 2: the effect of tetrandrine (also known as tetrandrine) alone on the biofilm of different Cryptococcus neoformans strains
[0057] Materials and methods
[0058] 1. Test drug
[0059] Tetrandrine: purchased from Sigma Company.
[0060] Fluconazole: purchased from Sigma Company.
[0061] Amphotericin B: purchased from Sangon Bioengineering Co., Ltd.
[0062] XTT: purchased from Sigma Corporation.
[0063] Menadione (menadione): purchased from Sigma Company.
[0064] Dimethyl sulfoxide (DMSO): China Pharmaceutical (Group) Shanghai Chemical Reagent Company, redistilled before use.
[0065] Acetone: purchased from Sigma Company.
[0066] Tetrandrine was made into 6.4mg / ml and 12.8mg / ml with dimethyl sulfoxide, amphotericin B was made into 4mg / ml with dimethyl sulfoxide, and fluconazole was made into 102.4mg / ml with dimethyl sulfoxide , and the test drug was stored at -20°C. Before the experiment, the drug stock solution was taken out and melted in a 35°...
Embodiment 3
[0097] Embodiment 3: the effect of tetrandrine (also known as tetrandrine) alone on the biofilm of different Aspergillus strains
[0098] Materials and methods
[0099] 1. Test drug
[0100] Tetrandrine: purchased from Sigma Company.
[0101] Fluconazole: purchased from Sigma Company.
[0102] Amphotericin B: purchased from Sangon Bioengineering Co., Ltd.
[0103] XTT: purchased from Sigma Corporation.
[0104] Menadione (menadione): purchased from Sigma Company.
[0105] Dimethyl sulfoxide (DMSO): China Pharmaceutical (Group) Shanghai Chemical Reagent Company, redistilled before use.
[0106] Acetone: purchased from Sigma Company.
[0107] Tetrandrine was made into 6.4mg / ml and 12.8mg / ml with dimethyl sulfoxide, amphotericin B was made into 4mg / ml with dimethyl sulfoxide, and fluconazole was made into 102.4mg / ml with dimethyl sulfoxide , and the test drug was stored at -20°C. Before the experiment, the drug stock solution was taken out and melted in a 35°C incubator,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com