Sustained release preparation of dronedarone hydrochloride
A dronedarone hydrochloride and slow-release preparation technology, which is applied in the field of medicine, can solve the problems of affecting the steady-state blood drug concentration of the drug, inconvenient use for patients, and short biological half-life, so as to achieve less medication times, high safety, and bioavailability high degree of effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
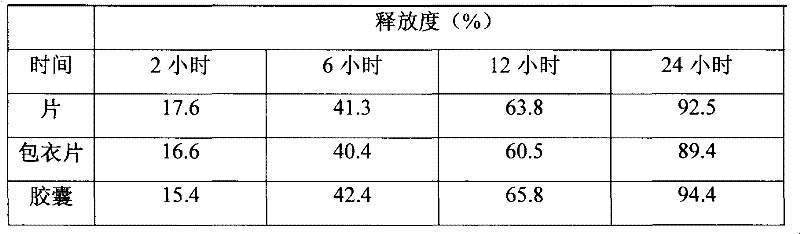
[0031] The sustained-release preparation containing dronedarone hydrochloride as an active ingredient of the present invention is further specifically described by the following examples, but it is not intended to limit the present invention.
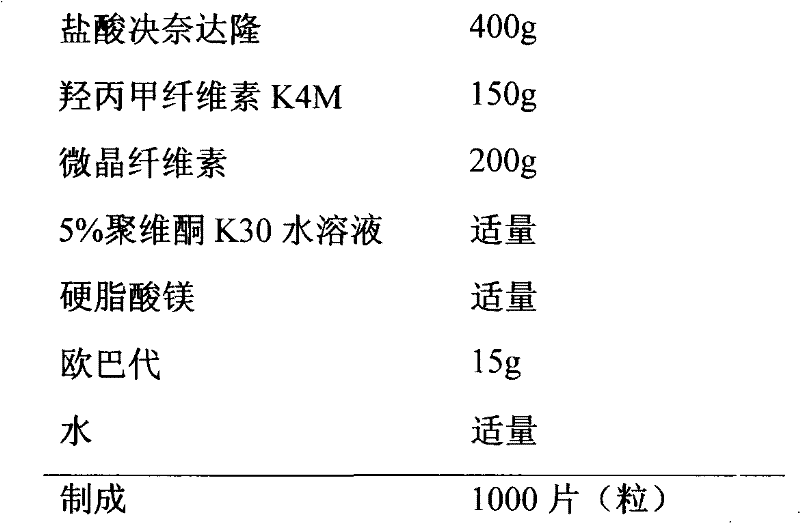
[0032] prescription
[0033]
[0034] Preparation
[0035] (1) Preparation of granules: Hypromellose and microcrystalline cellulose were sieved separately and mixed evenly. Then add dronedarone hydrochloride, mix well, use 5% povidone K3O aqueous solution as binder to make soft material, sieve with 20 mesh to prepare wet granules, dry at 50°C, sieve with 18 mesh for granulation, and set aside.
[0036] (2) Preparation of coating liquid: add Opadry to pure water, and add pure water to 100ml, stir for 1 hour, set aside.
[0037] (3) Coating the granules of (1) to obtain coated granules.
[0038] (4) Add an appropriate amount of magnesium stearate to the granules obtained in (3), mix well, and press into tablets to obtain tablets.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com