Decitabine lyophilized preparation and preparation method thereof
A freeze-dried preparation, decitabine technology, applied in the field of decitabine freeze-dried preparation and its preparation, can solve the problems of low solubility, unstable and easy degradation of decitabine, etc. The effect of excellent quality and high safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] The preparation method of decitabine freeze-dried preparation of the present invention comprises the following steps:
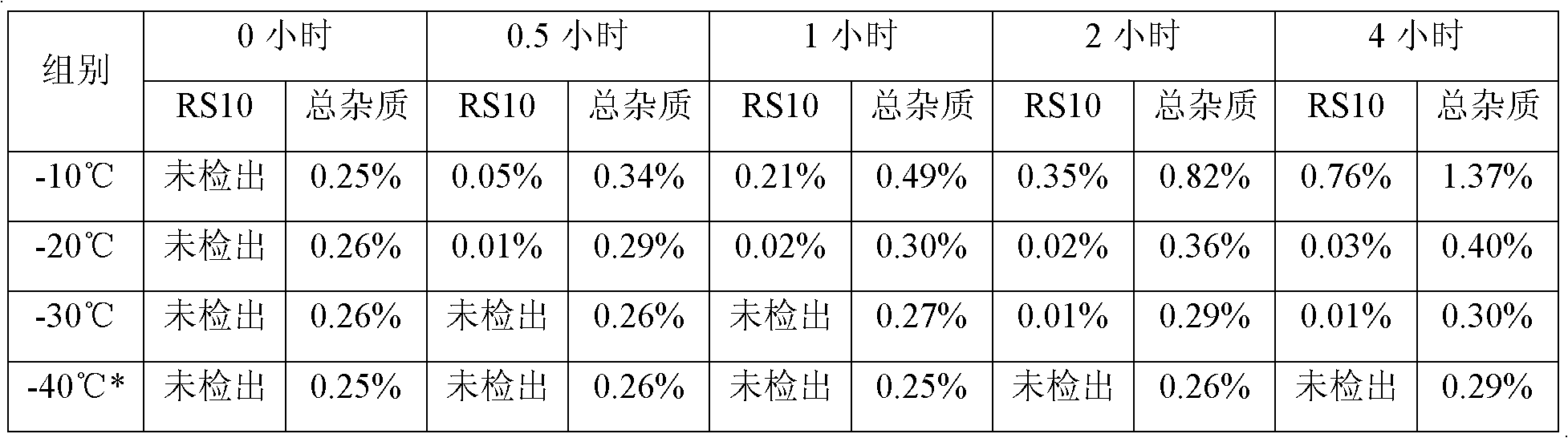
[0030] (1) Dissolving decitabine in a mixed solvent composed of dimethyl sulfoxide and a water-miscible organic solvent, and freezing to below -30°C; the organic solvent is selected from methanol, ethanol, isopropyl Alcohol, n-butanol, tert-butanol;
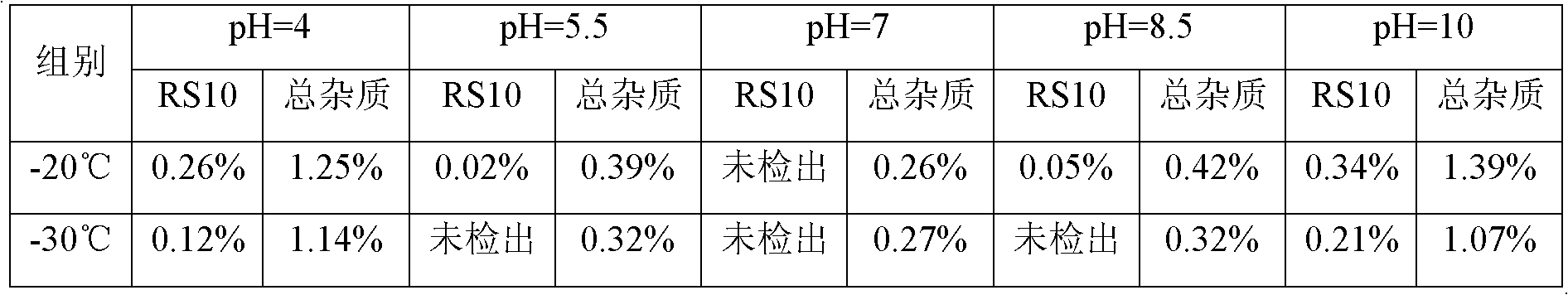
[0031] (2) Dilute the solution obtained in step (1) with water for injection in which the pH regulator is dissolved to prepare a mixed solution, filter, and subpackage. The temperature during this operation needs to be controlled at -20 ~ -30°C, so as to facilitate the control of the mixed solution at - 20~-30℃;
[0032] (3) Freeze-drying is made into freeze-dried preparation;
[0033] Wherein, in the mixed solution described in step (2): dimethyl sulfoxide accounts for 5% to 20% of the total volume of the mixed solution, and the "water-miscible organic solvent" accounts for 10 to 35% of the total volum...
Embodiment 1
[0062] prescription
[0063]
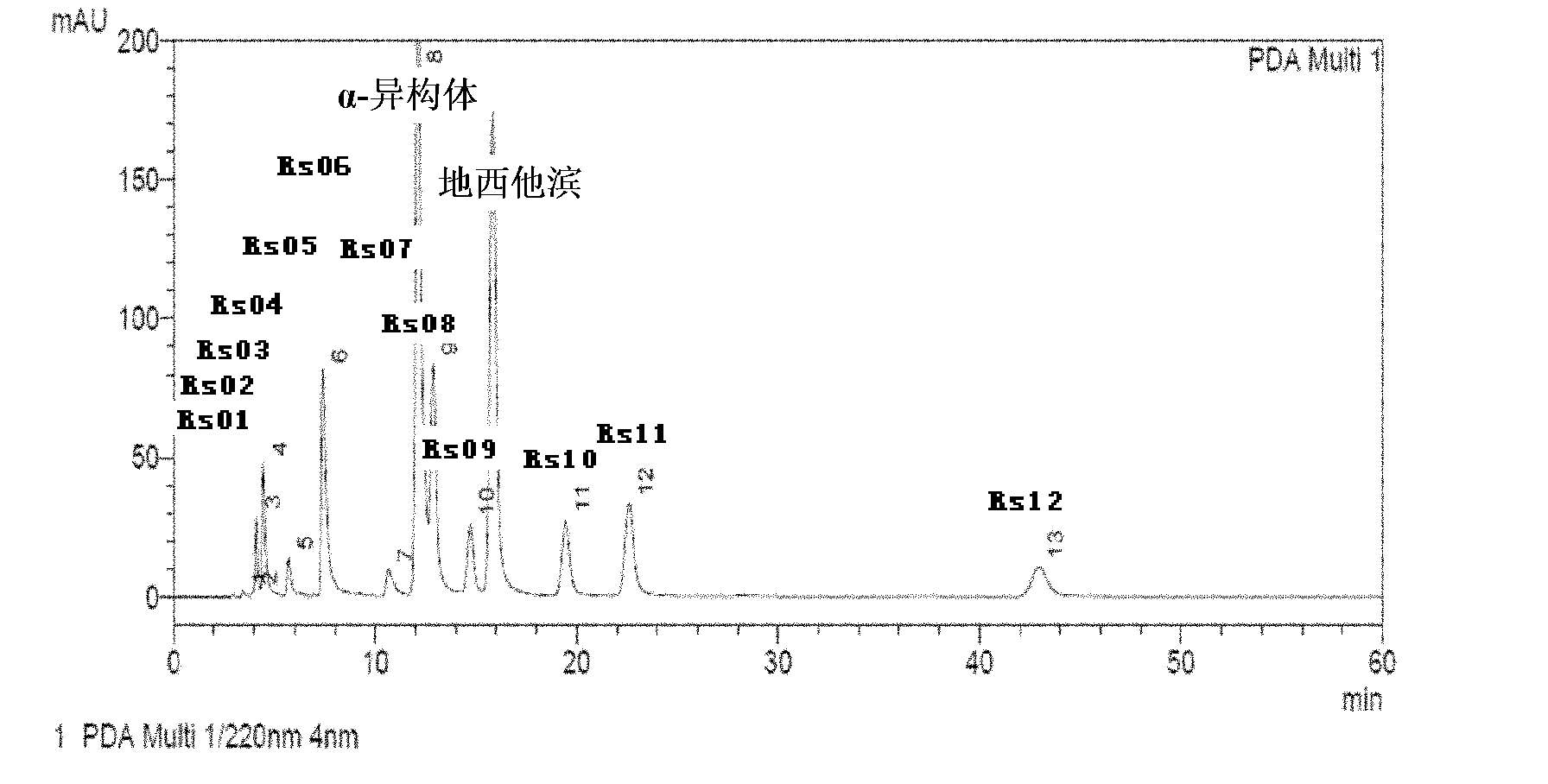
[0064] Precisely weigh decitabine, dissolve it with dimethyl sulfoxide, mix it with ethanol, and freeze it to about -30°C; prepare a water solution for injection containing potassium dihydrogen phosphate and sodium hydroxide in the prescribed amount, and refrigerate it until 2- After 5°C, mix with the above-mentioned organic solvent in which decitabine is dissolved, stir to dissolve, keep warm at -30~-25°C, filter through a 0.22 micron filter membrane, pack in vials, freeze-dry, and wait for After the solvent of the finished product is completely removed, the stopper is pressed, and the cover is tied, and the product is obtained. After inspection, the RS10 content of the finished product is 0.02%, the total impurities of the finished product are 0.48%, and the content of dimethyl sulfoxide is 0.01%.
Embodiment 2
[0066] prescription
[0067]
[0068] Precisely weigh decitabine, add dimethyl sulfoxide and ethanol to dissolve, and freeze the solvent at -30°C; prepare a water-for-injection solvent containing prescription potassium dihydrogen phosphate and sodium hydroxide, and refrigerate to 2-5°C , mix the prepared two solvents, stir to dissolve, keep warm at -25°C to -20°C, after passing the intermediate inspection, filter through a 0.22 micron filter membrane, pack in cillin varieties, freeze-dry, and wait for the finished product After the solvent is completely removed, the plug is pressed, and the cover is tied, and the product is obtained. After inspection, the RS10 content of the finished product is 0.02%, the total impurities of the finished product are 0.50%, and the content of dimethyl sulfoxide is 0.01%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com