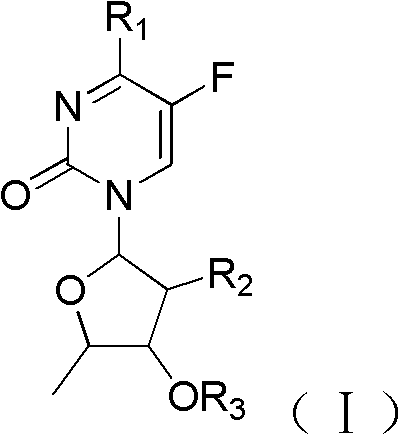
Floxuridine compound and preparation method and application thereof
A technology of floxuridines and compounds, which is applied in the field of medicine and can solve problems such as limited application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
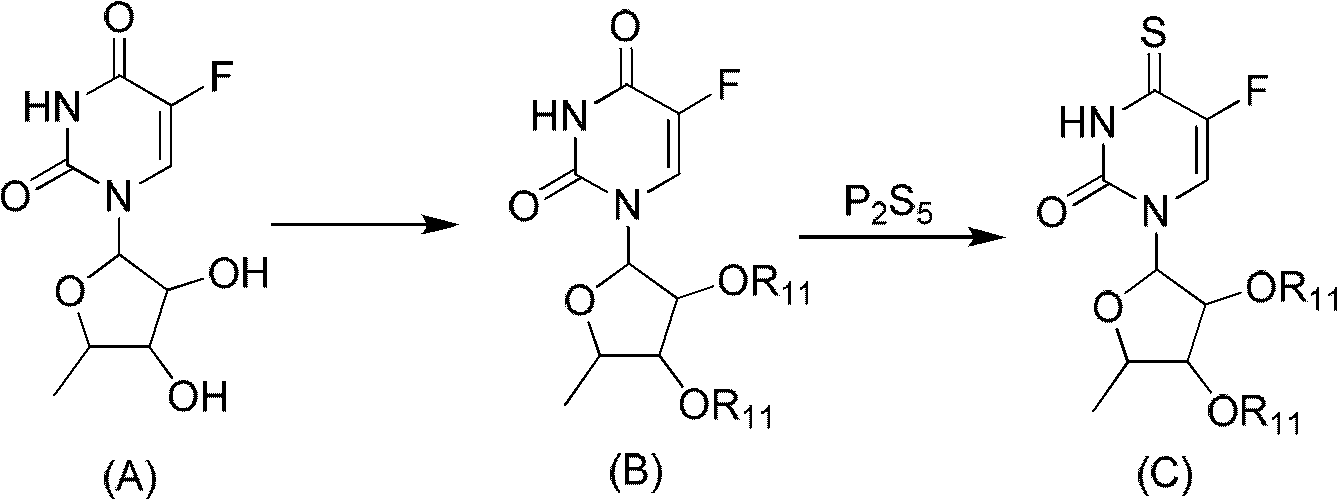
[0046] Preparation of 5'-deoxy-5-fluoro-thiouridine (compound 1)
[0047] A, 2', 3'-di-O-acetyl-5'-deoxy-5-fluorouridine (compound of general formula B, wherein R 9 for acetyl)
[0048] With reference to the literature method (Dong Hui et al. Chinese Journal of Pharmaceutical Industry, 33:108 (2002) to obtain the starting material 5'-deoxy-5-fluorouridine (compound A). Get 2g (8.1 mmol) of compound A and dissolve it in 40ml without In water pyridine, add dropwise 1.8ml (18 mmol) acetic anhydride, and stir overnight at room temperature. Then add 5ml methyl alcohol and continue to stir for 15 minutes, and the solution is evaporated to dryness. The solid is dissolved in 40ml dichloromethane, and is dissolved in 40ml of 10% sodium bicarbonate aqueous solution Wash, and then extract the aqueous phase with 10ml of dichloromethane × 2. Combine the dichloromethane liquids, dry over anhydrous sodium sulfate, filter, and evaporate to dryness. The residue is recrystallized with 10ml of ...
Embodiment 2
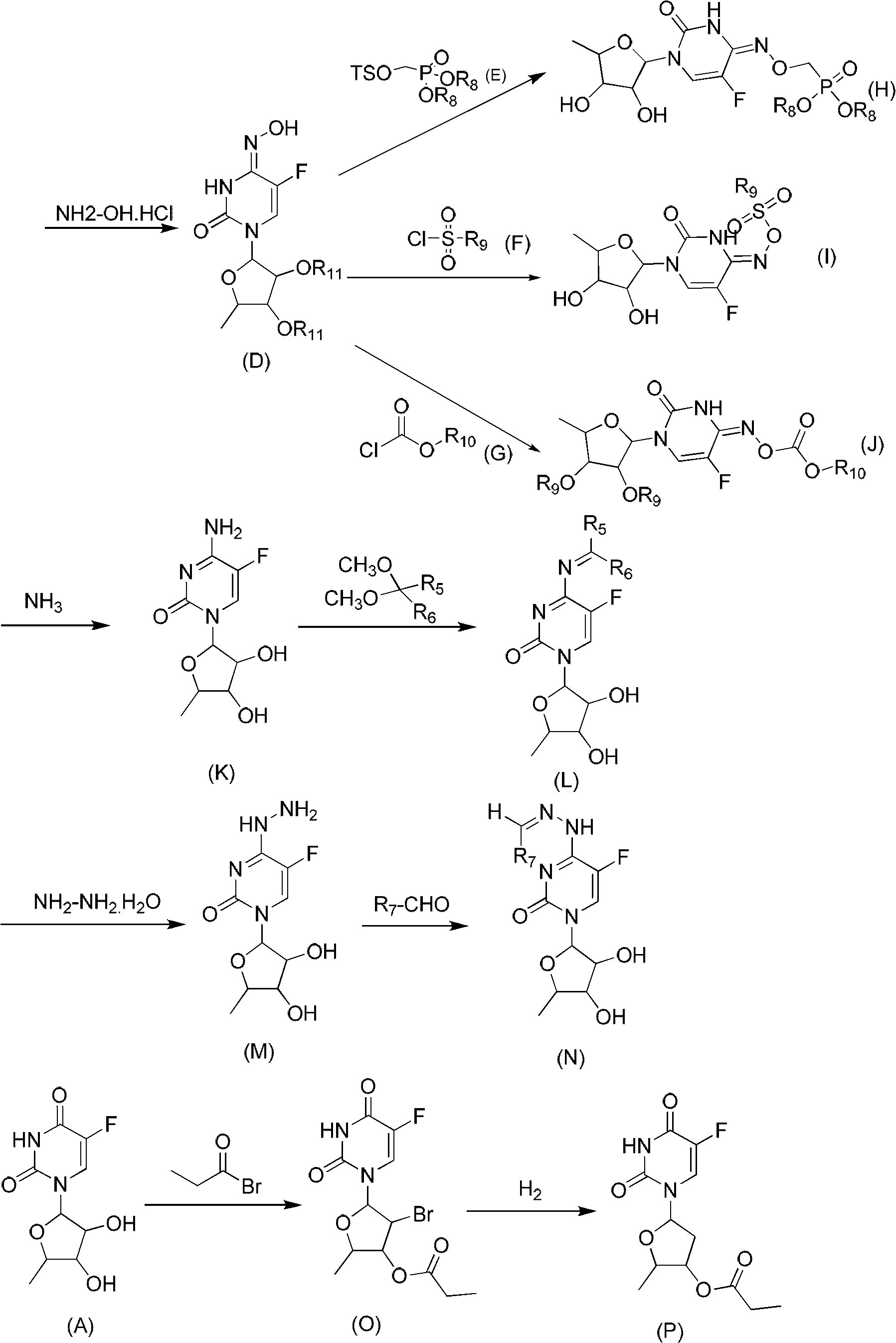
[0054] Preparation of 5'-deoxy-5-fluoro-N 4 -Oximocytidine (compound 2)
[0055] A, 2', 3'-di-O-acetyl-5'-deoxy-5-fluoro-N 4 -Hydroxycytidine (compound of general formula D)
[0056] Get 2g (5.8 mmol) 2', 3'-di-O-acetyl-5'-deoxy-5-fluoro-thiouridine and join in 30ml ethanol, then add 0.48g (7 mmol) hydroxylamine hydrochloride and 0.55g (8 mmol) of potassium carbonate, stirred at 60°C for 10h, and evaporated to dryness. The residue was dissolved in 100ml ethyl acetate, washed with 50ml×2 10% HCl aqueous solution, and the aqueous phase was extracted with 50ml ethyl acetate. The ethyl acetate phases were combined, dried over anhydrous sodium sulfate, filtered and evaporated to dryness. The residue can be directly used in the next reaction without purification.
[0057] B. 5'-deoxy-5-fluoro-N 4 -Oximocytidine (compound 2)
[0058] Take 2g (5.8 mmol) of 2',3'-di-O-acetyl-5'-deoxy-5-fluoro-N 4 -Oxime cytidine was dissolved in 30 ml tetrahydrofuran and then 10 ml methanol and...
Embodiment 3
[0060] Preparation of 5'-deoxy-5-fluoro-N 4 -aminocytidine (compound 3)
[0061] Take 2g (7.7 mmol) of 5'-deoxy-5-fluoro-thiocytidine, dissolve it in 50ml of methanol, cool to 0°C, slowly drop into 3ml of 50% hydrazine hydrate solution, stir for half an hour and use The 732 type cation exchange resin is neutralized to about pH=7. The resin was filtered off, the solution was evaporated to dryness, and the residue was purified by a silica gel column to obtain 1.4 g of a solid with a yield of 69.6%. 1 H-NMR (DMSO-d 6 )δ (ppm): 1.21 to 1.22 (3H, d, 4'CH 3 ), 3.60 to 3.63 (1H, d, 3'-H), 3.73 to 3.77 (1H, d, 4'-H), 3.98 to 4.01 (1H, d, 2'-H), 5.00 (1H, s, OH), 5.22 (1H, s, OH), 5.61 to 5.64 (1H, d, 1'-H), 7.16 (1H, d, CHCF), 10.12 (1H, s, NH)
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com