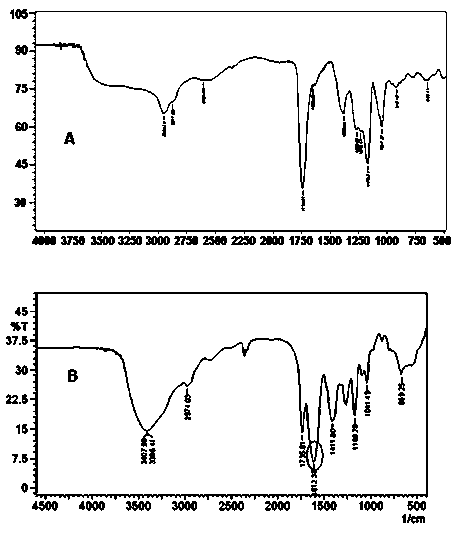
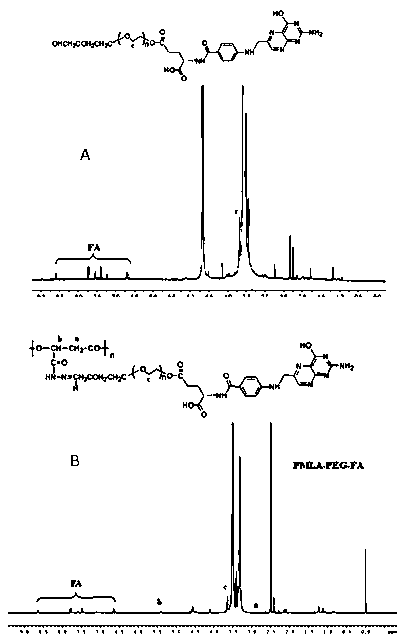
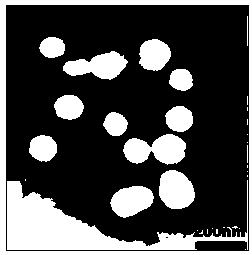
Multifunctional poly(malic acid) carried drug for targeting treatment of tumors
A technology of polymalic acid and anti-tumor drugs, which can be used in anti-tumor drugs, drug combinations, pharmaceutical formulations, etc., and can solve problems such as the limitation of micelle stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1: TAT-PEG 2 -PMLA(DOX)-Hz-PEG 6 -Establishment of FA drug loading system
[0040] (1) Aldehyde polyethylene glycol-folate (CHO-PEG 6 -FA) Preparation
[0041] Weigh 44 mg of folic acid (FA) and dissolve it in an appropriate amount of DMSO, and add 22 mg (Boc) in turn 2 O, 20 mg triethylamine, reacted at room temperature in the dark for 4 h to obtain Boc-protected FA; then sequentially added 170 mg HO-PEG 6 -CHO (M n : 6000), 19 mg EDC·HCl, 10 mg DMAP, protected from light at room temperature, stirred overnight; the reaction solution was dialyzed with DMSO for 24 h, deionized water for 48 h, and freeze-dried to obtain CHO-PEG-FA(Boc); CHO-PEG-FA(Boc) was dissolved in 10 mL of anhydrous CH 2 Cl 2 5 mL TFA was added dropwise, stirred at room temperature for 4 h, and CH was removed by rotary evaporation. 2 Cl 2 and TFA to give FA-PEG-CHO.
[0042]
[0043] (2) Polymalic acid (PMLA-NH 2 ) preparation
[0044] Weigh 80 mg PMLA, dissolve 88 mg NHS in 20...
Embodiment 2
[0054] Embodiment 2: TAT-PMLA (CPT-NH 2 )-Hz-PEG 6 Establishment of drug loading system
[0055] Weigh a slightly excess amount of polymalic acid (PMLA; Mn: 5,000) 30 mg and dissolve it in an appropriate amount of DMF, add EDC?HCl 100 mg, 2 ml 5% hydrazine hydrate, and stir overnight at room temperature. Add 200mg OHC-PEG 6 -OH (Mn: 6,000) was stirred at room temperature for 10 h. After the end, dialyze with deionized water for 48h to remove unreacted substrate and solvent, and freeze-dry to obtain the product PMLA-Hz-PEG 6 -OH.
[0056] The PMLA-Hz-PEG 6 -OH was dissolved in an appropriate amount of DMF, EDC?HCl and TEA were added, and stirred in an ice-water bath. The DMF solution of appropriate amount of TAT is added dropwise in this system, then adds 50mg aminocamptothecin (CPT-NH 2 ), reacted at 0°C for 2 hours, then transferred to room temperature for 24 hours, dialyzed in deionized water for 24 hours, and freeze-dried to obtain yellow powder TAT-PMLA (CPT-NH 2 )...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com