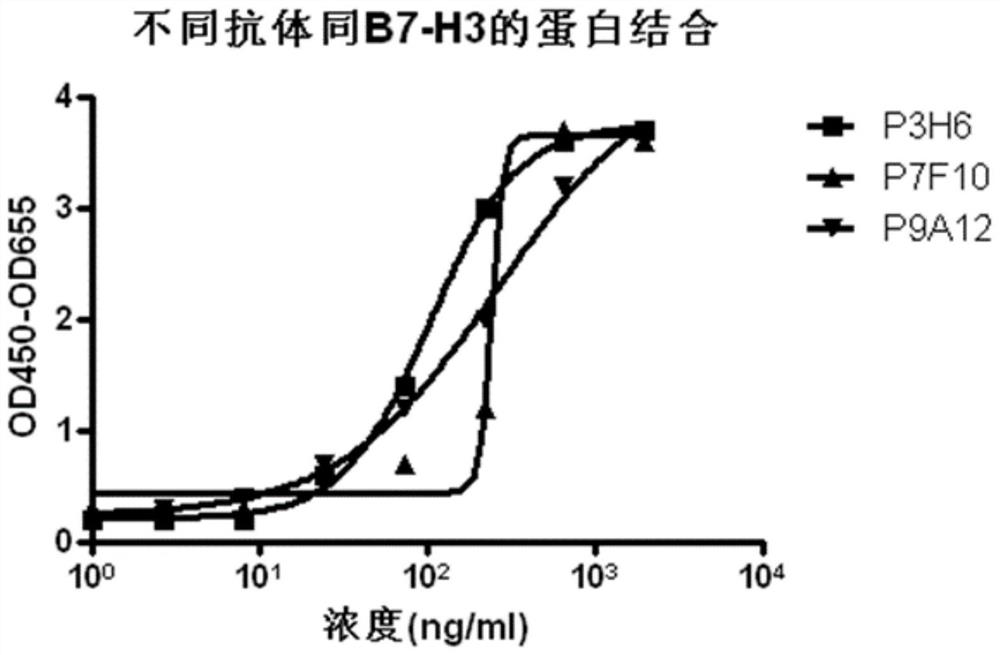
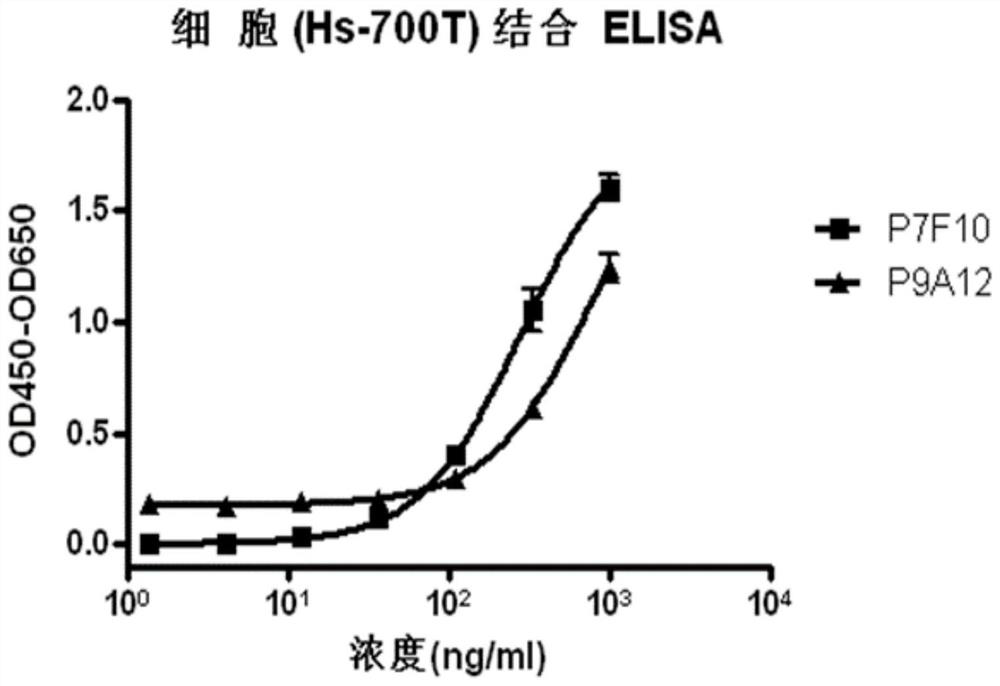
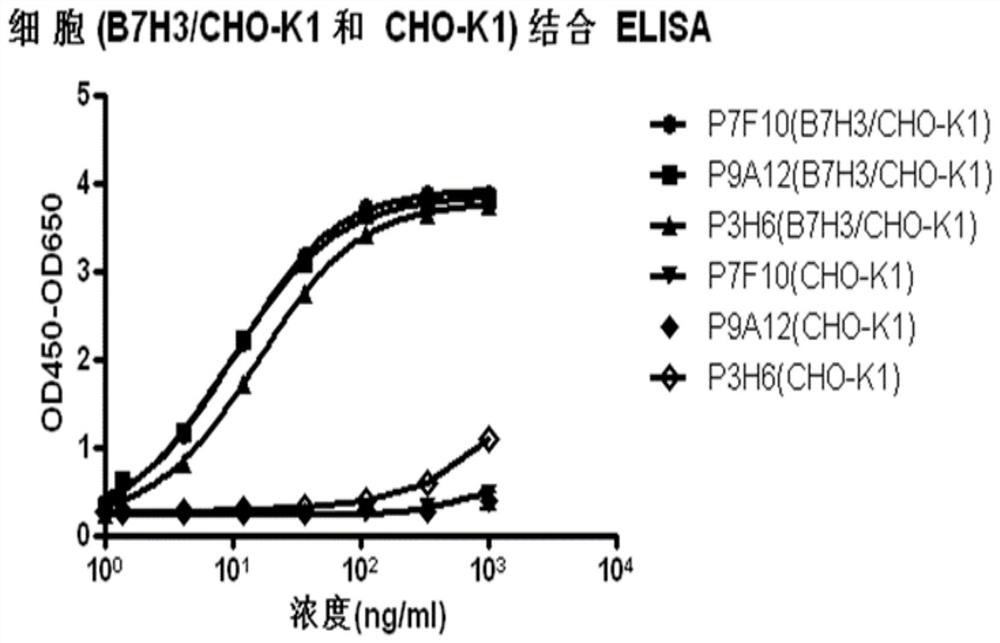
Anti-B7-H3 antibody as well as preparation method, conjugate and application thereof
A B7-H3, immunoconjugate technology, applied in chemical instruments and methods, anti-animal/human immunoglobulin, anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, etc. Tumor efficacy needs to be further improved, immunogenicity risk and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] Preparation of Example 1 Human B7-H3 Protein
[0083] Select the nucleic acid sequence of amino acids 29-245 of human 2Ig B7-H3, in which a purified 10His tag is added to the N-terminal and a detection Myc tag is added to the C-terminal, named H2M; the nucleic acid sequence of amino acids 27-461 of human 4Ig B7-H3 is selected , where the N-terminus adds a purification tag 10His and the C-terminus adds a detection Myc tag, named H4M; select the nucleic acid sequence of amino acids 27-461 of human 4Ig B7-H3, wherein the N-terminus adds a detection myc tag and the C-terminus adds a purified 10His tag , named M4H. The gene plasmids H2M-pUC57, M4H-pUC57, and H4M-pUC57 of the above three B7-H3 antigens were synthesized, respectively, and pv81 expression vector plasmids were synthesized, respectively digested by EcoRI and SmalI, and ligated to transform Escherichia coli competent cells Trans-T1, The correct clones were screened and amplified by PCR to extract a large number o...
Embodiment 2
[0085] Example 2 Library Preparation
[0086]Take 1ml library Lambda size 2.91×10 9 and the library Kappa size is 3.72×10 9 Add 2.0L fresh culture medium 2YT+100μg / ml Amp+2% glucose, the initial OD600 of the library bacteria solution is less than 0.1, and culture at 37°C and 200rpm. When the OD600 is 0.5-0.6, add 365 μL of M13K07 with a titer of 9.6×10 12 / ml, the amount added is 10 times the amount of bacteria, the amount of bacteria = OD600 × 8.0 × 10 8 / ml×Shaking bacteria volume, assisting phagemid infection. After adding helper phage, culture at 37°C for 30min, then at 200rpm for 30min, and finally at 4000rpm for 10min, resuspend with 2.0L equal volume of 2YT+100μg / mlAmp+50μg / ml Kana, and culture at 30°C 16h, for expression. After the expression culture was completed, the bacterial solution was taken and centrifuged at 8000 rpm for 30 minutes at 4°C, and the supernatant was removed. Centrifuge at high speed, remove the bacteria, add 1 / 5 volume of PEG / NACL to the sup...
Embodiment 3
[0087] Example 3 Anti-B7-H3 Antibody Phage Library Screening
[0088] Liquid-phase panning: 150 μL magnetic beads Dynabeads M-280 were blocked with 1% casein at room temperature for 1 hour, then 100 μL of the prepared phage library Phage was added, shaken gently, and blocked for 1 hour at room temperature. After the completion of blocking, add 30 μg of biotinylated B7-H3 antigen H4M and incubate at room temperature for 1 h. After the incubation is completed, the complex of antigen H4M and phage antibody is incubated with the blocked magnetic beads for 15 min to make the complexes bind to the magnetic beads. Wash with PBST and PBS 15 times respectively. Then add 1ml of trypsin (10μg / ml) to elute the phages bound to the antigen, the elution volume is 1ml, infect TG1 which is growing in the logarithmic phase, measure the titer, and amplify the phages for the next round of panning . A total of 3 rounds of panning were performed, and the last 2 rounds of panning were the same as ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com