Method for recovering rare earth elements from waste red phosphor
A red phosphor, rare earth element technology, applied in rare earth metal compounds, chemical instruments and methods, inorganic chemistry, etc., can solve problems such as rare earth recycling not involved
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
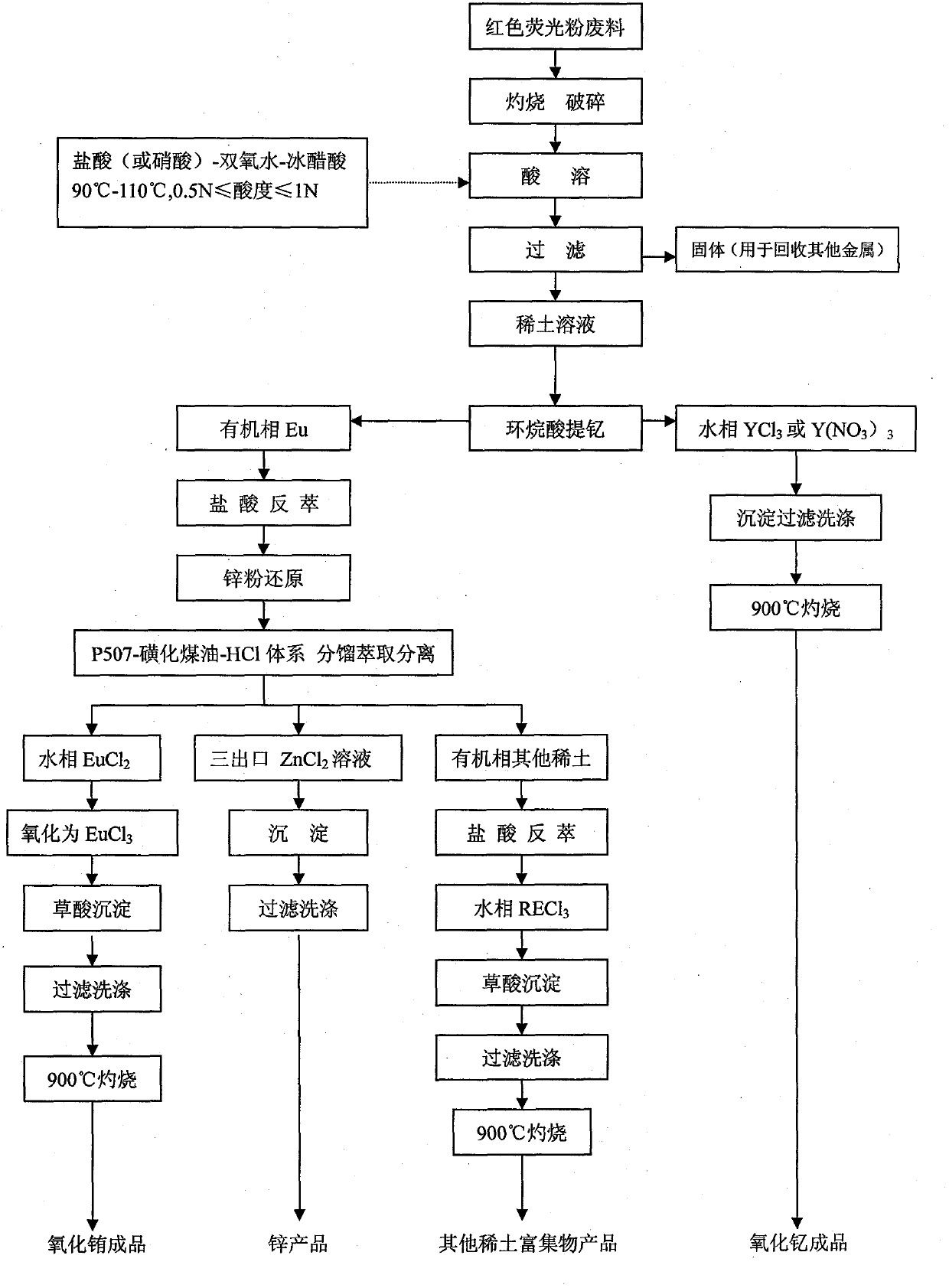
[0017] Take 1000g of discarded red phosphors for three primary color fluorescent lamps, total rare earth (TREO): 81.56%, Eu 2 O 3 / TREO: 7.25%, Y 2 O 3 / TREO: 92.73%, other rare earth 2 O 2 )(3%)-glacial acetic acid (CH 3 COOH) (8%), amount of rare earth metal: volume of composite acid = 1:3, acid dissolution conditions are: acidity 0.5N, acid dissolution temperature 90℃, after acid dissolution, filter to remove insolubles to obtain 2.4L concentration of 338.1g / l The rare earth chloride solution, the calculated yield of rare earth dissolution is 99.5%. Dilute the rare earth chloride solution to a concentration of 120±5g / l and adjust the pH to 2.5±0.3, and enter the fractionation extraction system of 22% naphthenic acid + 18% mixed alcohol + 60% sulfonated kerosene in the organic phase saponified with NaOH After extraction and separation, after 75-stage extraction and 15-stage washing, high-purity yttrium chloride (YCl 3 ) Solution, the solution is precipitated and filtered with ...
Embodiment 2
[0019] Take 1000g of discarded red phosphors for tri-color fluorescent lamps, total rare earth (TREO): 81.56%, EU 2 O 3 / TREO: 7.25%, Y 2 O 3 / TREO: 92.73%, other rare earth 2 O 2 )(5%)-glacial acetic acid (CH 3 COOH) (3%), amount of rare earth metal: volume of composite acid = 1:3, acid dissolution conditions: acidity 0.5N, acid dissolution temperature 110°C, after acid dissolution, filter to remove insoluble matter to obtain 2.41L with a concentration of 337.0g / l The rare earth chloride solution, the calculated yield of rare earth dissolution is 99.6%. The subsequent process steps are the same as in Example 1.
Embodiment 3
[0021] Take 1000g of discarded red phosphors for three primary color fluorescent lamps, total rare earth (TREO): 81.56%, Eu 2 O 3 / TREO: 7.25%, Y 2 O 3 / TREO: 92.73%, other rare earth 3 )(89%)-oxidant(H 2 O 2 )(3%)-glacial acetic acid (CH 3 COOH) (8%), amount of rare earth metal: volume of composite acid = 1:3, acid dissolution conditions are: acidity 0.5N, acid dissolution temperature 90℃, after acid dissolution, filter to remove insolubles to obtain 2.41L concentration of 337.0g / l The calculated rare earth nitrate solution yield is 99.6%. Dilute the rare earth nitrate solution to a concentration of 120±5g / l and adjust the pH to 2.5±0.3, and enter it into a fractional extraction system of 22% naphthenic acid + 18% mixed alcohol + 60% sulfonated kerosene in the organic phase saponified with NaOH Extraction and separation, after 75-stage extraction and 15-stage washing, high-purity yttrium nitrate (Y(NO 3 ) 3 ) Solution, the solution is precipitated and filtered with high-quality...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com