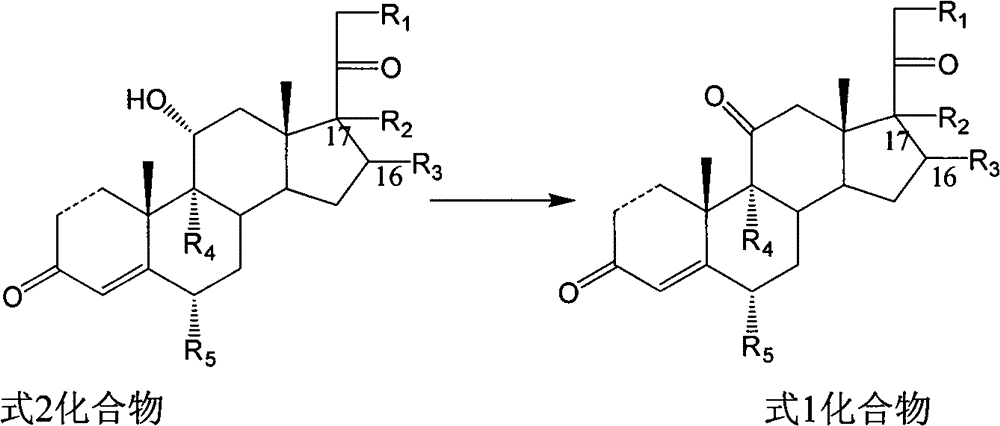
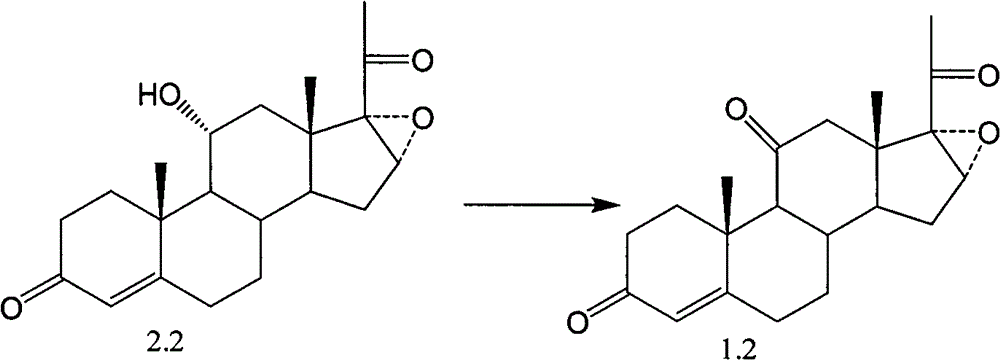
Novel technology for oxosynthesis of pregnane 11-bit ketonic group
A compound, nitrogen oxide free radical technology, applied in the field of synthesis of pregnane 11-position ketone group, can solve problems such as multiple impurities and environmental protection problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
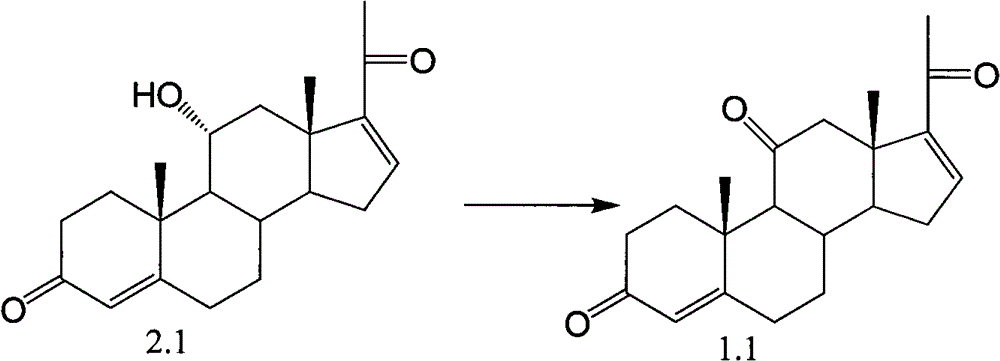
Embodiment 1
[0060]
[0061]Add 10mmol of compound 2.1 into 30ml of dichloromethane, add 12mmol of NaOCl in 20ml of aqueous solution while stirring, then add 0.15mmol of TEMPO, stir well at 20°C, react until there is no raw material after 4 hours, add saturated NaOCl 2 SO 3 The organic phase was washed several times with aqueous solution to remove TEMPO and inorganic salts. The organic layer was dried by adding anhydrous sodium sulfate, filtered, and then vacuum rotary evaporated to obtain the product after removing the organic solvent, yielding 9.0 mmol of 1.1 compound.
Embodiment 11
[0063] Add 10mmol of compound 2.1 into 30ml of dichloromethane, add 12mmol of NaOCl in 20ml of aqueous solution while stirring, then add 0.15mmol of TEMPO and 1mmol of NaBr, stir well at 20°C, react until there is no raw material after 1 hour, add saturated Na 2 SO 3 The organic phase was washed several times with aqueous solution to remove TEMPO and inorganic salts. The organic layer was dried by adding anhydrous sodium sulfate, filtered, and then vacuum rotary evaporated to obtain the product after removing the organic solvent, yielding 9.2 mmol of 1.1 compound.
Embodiment 12
[0065] Add 10mmol of compound 2.1 into 30ml of acetonitrile, add 12mmol of NaOCl in 20ml of aqueous solution while stirring, then add 0.15mmol of TEMPO and 1mmol of NaBr, stir fully at 20°C, react until there is almost no raw material after 2.5 hours, add saturated Na 2 SO 3 The organic phase was washed several times with aqueous solution to remove TEMPO and inorganic salts. The organic layer was dried by adding anhydrous sodium sulfate, filtered, and then vacuum rotary evaporated to obtain the product after removing the organic solvent, yielding 6.9 mmol of 1.1 compound.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com