Anti-egfr antibodies and their uses
A technology of antibody and humanized antibody, which is applied in anti-tumor drugs, anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, anti-animal/human immunoglobulin, etc., which can solve the problem of low antibody efficiency, Eliminate the low efficiency of killing cells, antigen variation and other issues, to achieve the effect of improving therapeutic efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
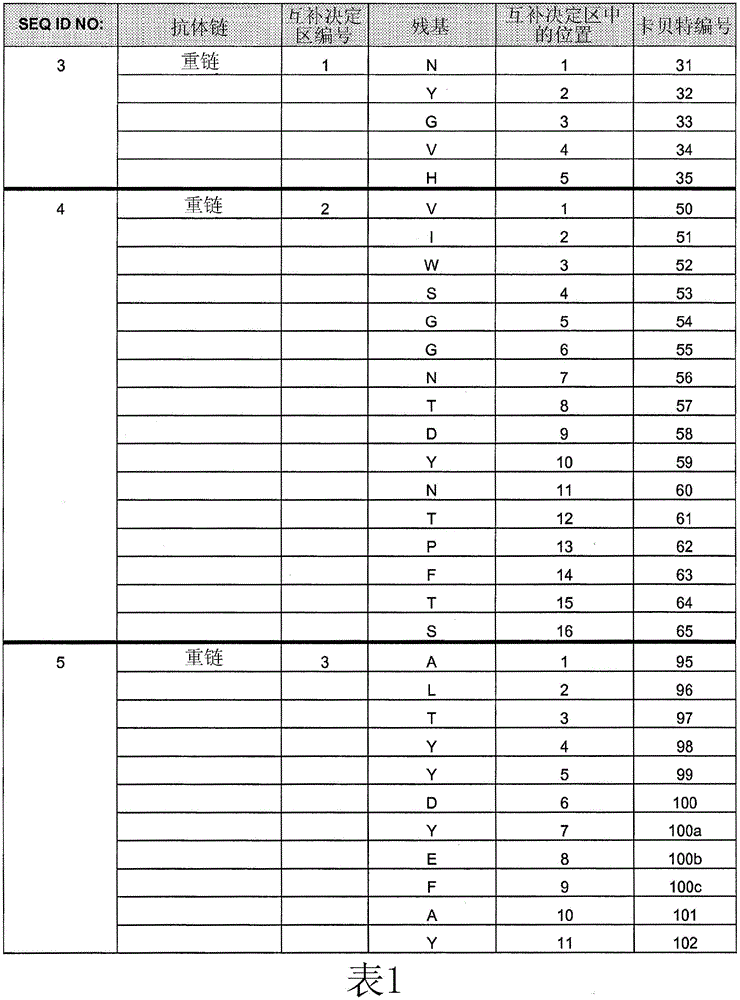
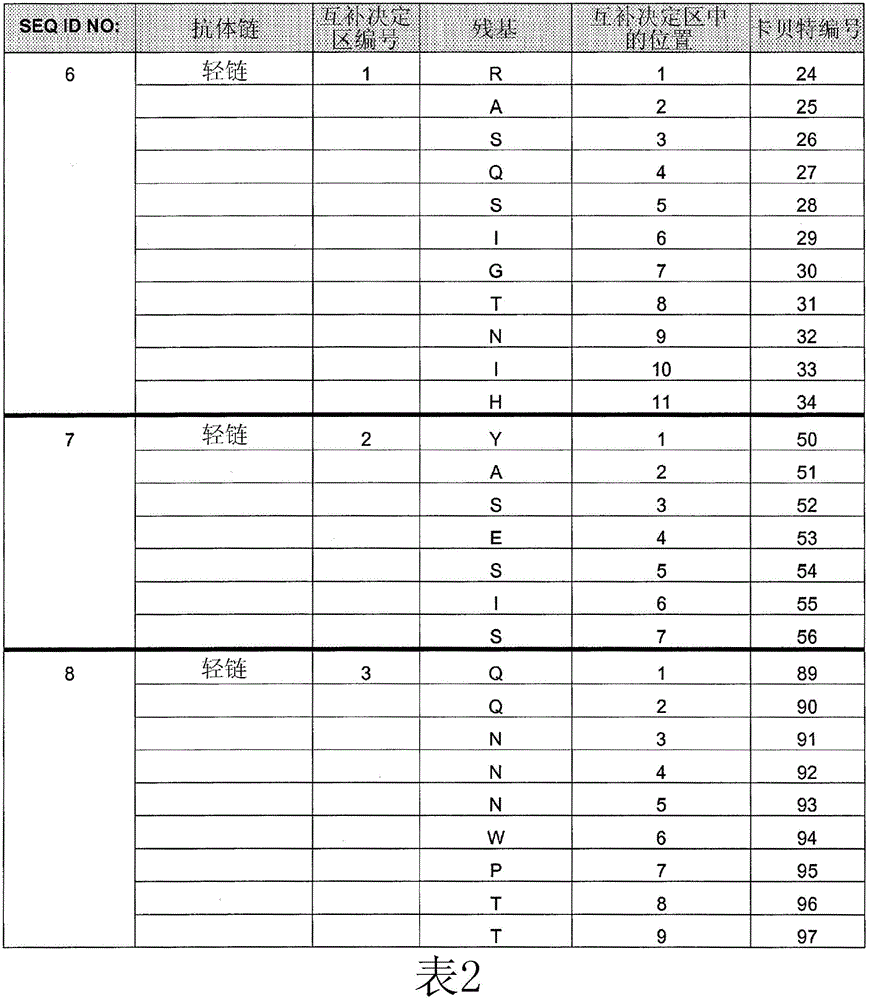
[0254] Example 1: Comparing the Binding Affinity of Cetuximab (ERBITUX) and HU225 to EGFR
[0255] The relative binding affinities of cetuximab and hu225 to EGFR were determined by competition assay using fluorescence activated cell sorting (FACS). A431 cells were grown to 2 × 10 on a V-bottom 96-well plate 5 cells / well. Cells were washed with FACS staining buffer (FSB). Starting with an initial concentration of 10 μg / mL of each competing antibody (unlabeled cetuximab and hu225), serial 1:3 dilutions were performed. Biotin-labeled cetuximab was diluted to a final concentration of 0.5 μg / mL (obtained from titration results). Next, biotin-labeled cetuximab was mixed with various concentrations of either competing antibody, and the mixture was transferred to a 96-well plate containing A431 cells. Plates were then incubated on ice for 1 hour, followed by two washes with FSB. 25 μL of streptavidin-RPE conjugate (Biosource) diluted to 2.5 μg / mL in FSB was added to each well and...
example 2
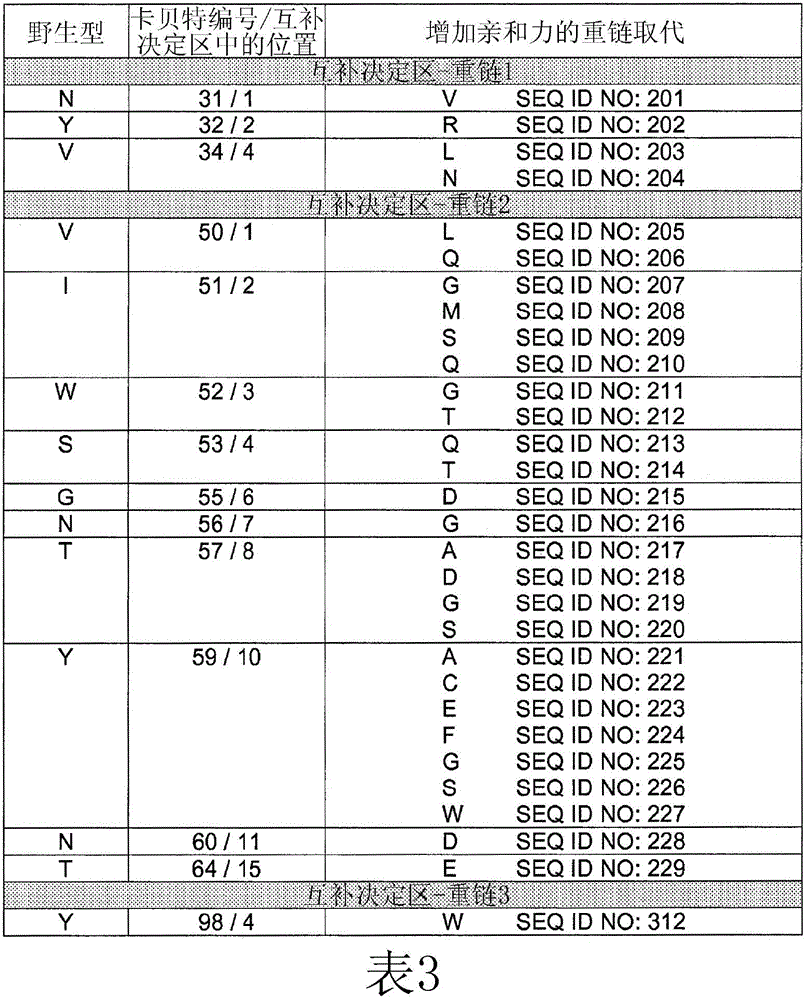
[0257] Example 2: Identification of HU225 variants with increased affinity for EGFR
[0258] Comprehensive mutation analysis was performed on the hu225 antibody to identify mutants with increased affinity for EGFR compared to wild-type hu225. Candidate high affinity mutants with increased affinity for EGFR compared to hu225 were analyzed by FACS to demonstrate relative increased binding to EGFR compared to hu225.
[0259] 1. Materials and Methods
[0260] To determine the binding of individual variants to EGFR, under subsaturated conditions (below K D ), and the degree of binding was quantified by FACS. Individual hu225 variants were constructed in mammalian cell surface display vectors (Akamatsu et al., 2007, J. Immunol. Methods 327(1-2):40-52) and transfected into in human cell lines. 50 μL of hybridoma-serum-free medium (SFM) containing 400 ng of plasmid DNA was mixed with 50 μL of hybridoma-SFM containing 1 μL of lipofectamine 2000 (Lipofectamine 2000), and incubated a...
example 3
[0265] Example 3: Further Characterization of HU225 Variants with Increased Affinity to EGFR (ELISA, ALPHALISA and BIACORE research)
[0266] 1. Materials and Methods
[0267] To determine the binding of individual variants to EGFR, under subsaturated conditions (below K D ), and the degree of binding was quantified by FACS (as described in Example 2 above), ELISA, alphaLISA and / or BIAcore.
[0268] ELISA involves the attachment of capture antibodies to a solid support, upon which an antigen-containing sample is added to a matrix or buffer suitable to minimize attachment to the solid phase. Enzyme-labeled antibodies are then added to detect and determine binding affinity. ELISA can be used to determine the binding affinity of individual variants (eg, anti-EGFR antibodies or antibody-binding fragments of the invention) to EGFR. See, eg, Patel et al., Anticancer Research 27, No. 5A:3355-3366, 2007; and Nix et al., "Immunoassays, a Practical Approach )”, J.P. Gosling, ed., pp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com