Stable antibody preparation
A preparation and stable technology, applied in the direction of antibodies, anti-tumor drugs, non-active ingredients of polymer compounds, etc., can solve problems such as unsatisfactory effects, and achieve the effect of guaranteeing shelf life, good stability, and preventing aggregation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1 Hyaluronidase and Cyclodextrin Effects on Antibody Preparations
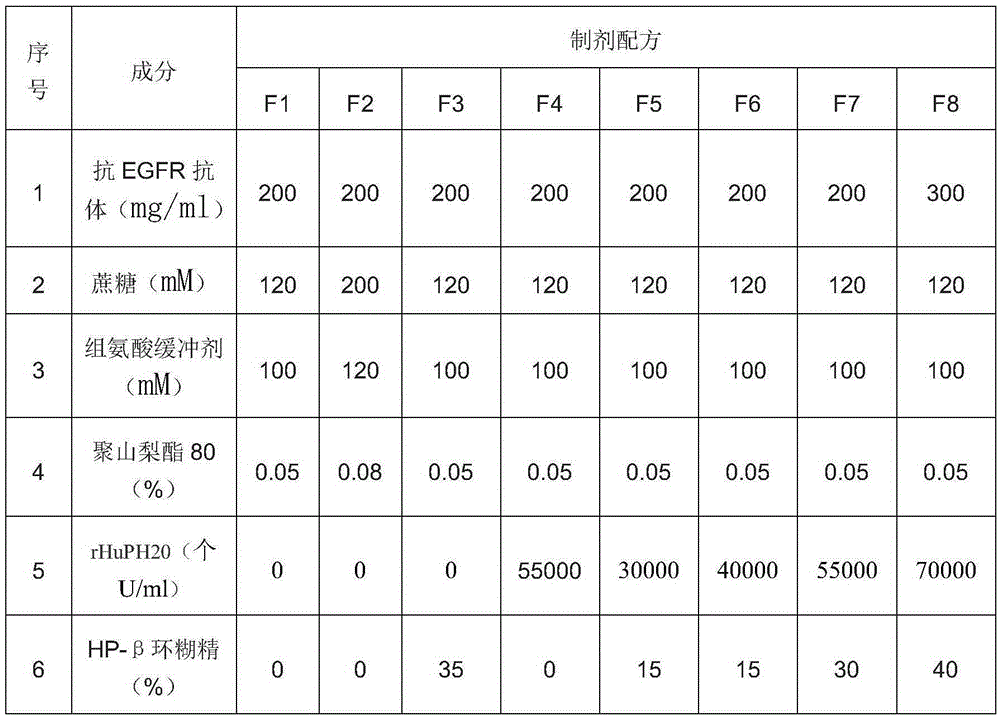
[0039] Stability study of anti-EGFR antibody formulations containing hyaluronidase and cyclodextrin at 25°C. The formulations may contain, in addition to hyaluronidase and cyclodextrin, further excipients as indicated in Table 1.
[0040] Table 1: Anti-EGFR antibody preparation formulation
[0041]
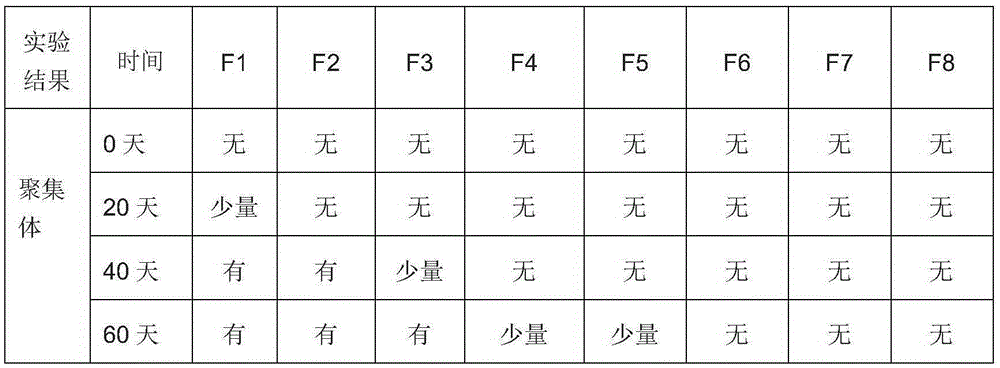
[0042] The above sample preparations were incubated at 35°C for 60 days, and then analyzed by SDS-PAGE at time points of 0, 20, 40, and 60 days, respectively. SDS-PAGE is used as an analytical technique to separate free and high molecular weight species from native proteins according to their molecular weight. Due to the high tendency of macromolecular antibodies to aggregate in high concentration antibody preparations, non-reducing SDS-PAGE was used to assess covalent aggregates.
[0043] Table 2: SDS-PAGE analysis results of aggregates of anti-EGFR antibody preparations with different formu...
Embodiment 2
[0046] The influence of embodiment 2 sugar on antibody preparation
[0047] The effect of different levels of sugar on the stability of anti-EGFR antibody formulations was evaluated. We placed the samples at -20°C, 8°C and 45°C under the conditions of 60% humidity and detected the aggregation of antibodies in anti-EGFR antibody preparations by SE-HPLC at different time points.
[0048] Table 3: Anti-EGFR antibody preparation formula table
[0049] serial number Element F9 F10 F11 F12 1 Anti-EGFR antibody (mg / ml) 200 200 200 200 2 Sucrose (mM) 20 100 200 400 3 Histidine buffer (mM) 150 150 150 150 4 Polysorbate 80(%) 0.02 0.02 0.02 0.02 5 rHuPH20 (unit U / ml) 50000 50000 50000 50000 6 HP-β cyclodextrin (%) 35 35 35 35
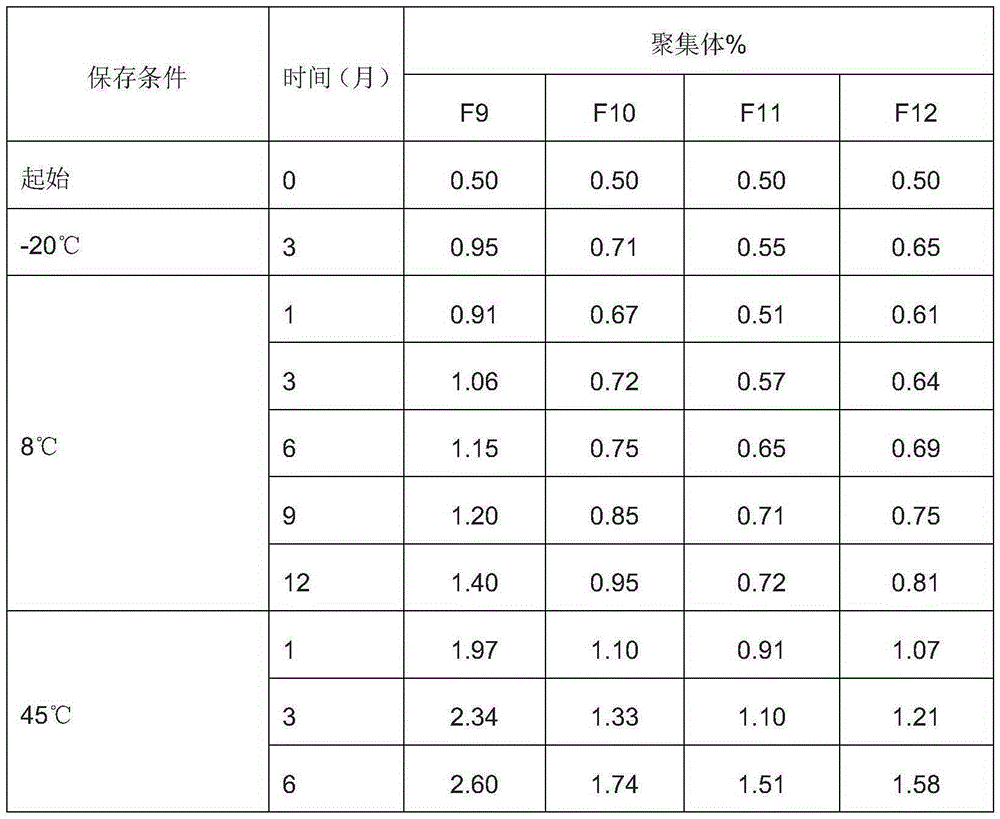
[0050] Table 4: Effects of sugars on the stability of anti-EGFR antibody preparations
[0051]
[0052] From the experimental results shown in Table 4, it can be seen that sugar h...
Embodiment 3
[0053] The impact of embodiment 3 surfactant on antibody preparation
[0054] The most suitable candidates in terms of stability were screened from the non-ionic surfactants polysorbates (eg polysorbate 20 or 80) and paloxamers (eg paloxamer 188 or 168). We mainly analyzed the aggregation of anti-EGFR antibody preparations with different contents of polysorbate 20 and paloxamer 188 during storage to analyze the influence of different surfactants on the stability of the preparation. We placed the samples under the conditions of 30°C and 60% humidity, and detected the aggregation of antibodies in the anti-EGFR antibody preparation by SE-HPLC method at different time points.
[0055] Table 5: Anti-EGFR antibody preparation formula table
[0056]
[0057] Table 6:: the impact of surfactant on the stability of anti-EGFR antibody preparations
[0058]
[0059] As can be seen from the above Table 6, compared with the preparation containing paloxamer 188 surfactant, polysorbat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com