Application of sanguinarine compound in preparation of drug for preventing and treating caner and pharmaceutical composition
A compound, the technology of sanguinarine, which is applied in the field of application and pharmaceutical composition of sanguinarine compounds in the preparation of drugs for the prevention and treatment of cancer, can solve problems such as complex components of sanguinarine, restrictions on the application of sanguinarine, and toxicity, etc. Achieve the effect of reducing the cost of cancer treatment, a considerable amount of resources, and a wide range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
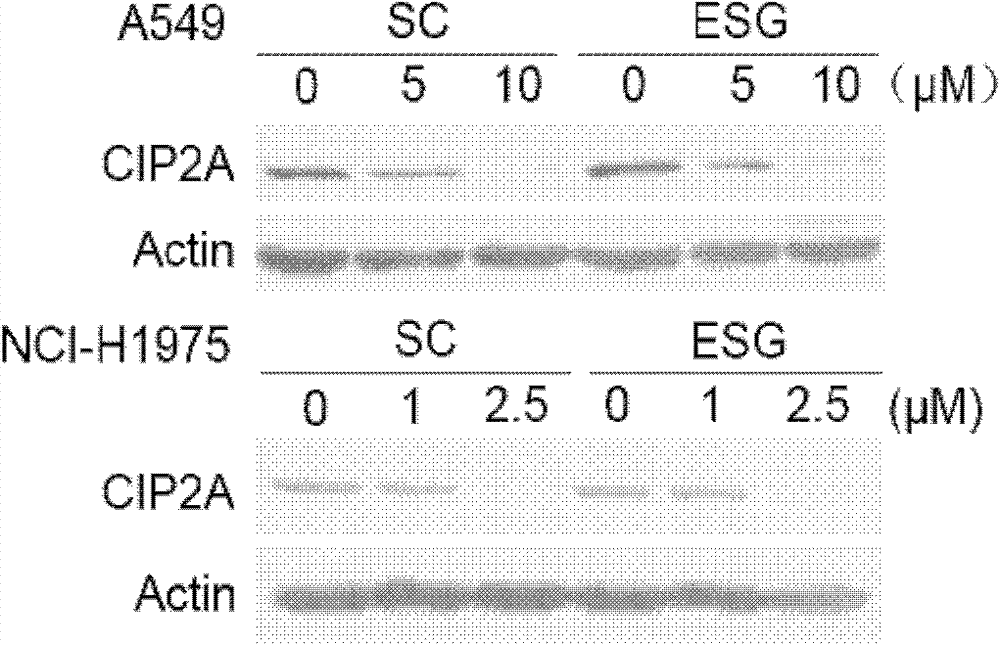
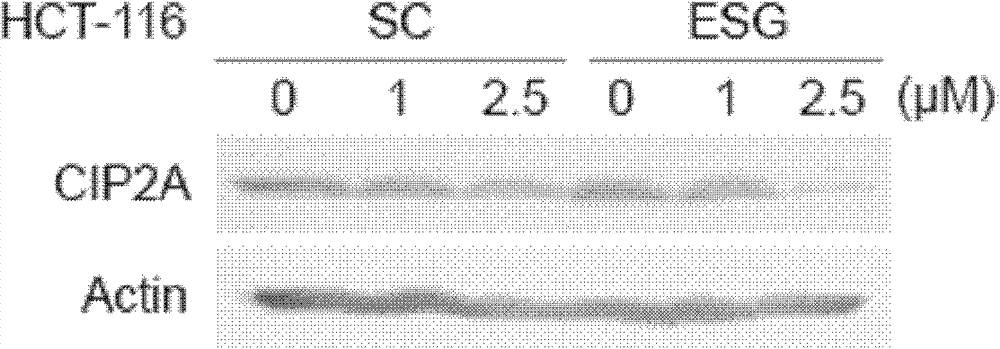
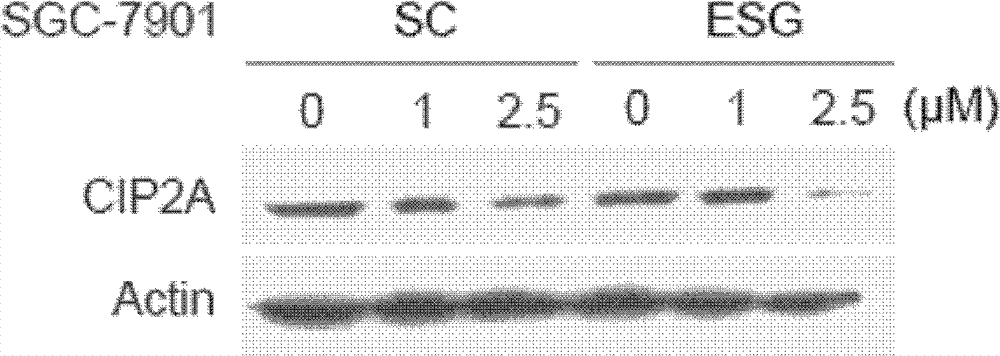
[0040] Various types of cancer cell lines A549, NCI-H1975, HCT-116, SGC-7901, MCF-7 and HepG2 were seeded in 6-well plates at a certain density, and after 24 hours, the cell fusion degree reached 70%- About 80%, A549 cells (0, 5, 10 μM), NCI-H1975 cells (0, 1, 2.5 μM), HCT-116 cells (0, 1, 2.5 μM), SGC cells were treated with different concentrations of SC and ESG, respectively. -7901 cells (0, 1, 2.5 μM), MCF-7 (0, 2.5, 5 μM) and HepG2 cells (0, 2.5, 5 μM) were treated, and after 24 hours, the cells were lysed with 1×SDS Loading Buffer to extract the total protein. The same amount of protein was subjected to Western Blot immunoblotting experiments, and anti-CIP2A (Santa Cruz) and anti-Actin (actin) antibodies (Sigma) were used to perform immunoblotting respectively to detect changes in their expression under drug treatment. The result is as Figure 1-5 As shown, in lung cancer cells A549 and NCI-H1975 ( figure 1 ), colon cancer cells HCT-116 ( figure 2 ), gastric cancer c...
Embodiment 2
[0042] 12 different types of cancer cell lines (A549, NCI-H1975, NCI-H460, HCC827, SPC-A-1, Glc-82, L78, 95D, HCT-116, SGC-7901 , MCF-7 and HepG2 cells) were inoculated in a 96-well plate (about 5000 cells per well, 100 μl medium), cultured (37°C, 5% CO 2 Incubator) 24 hours later, the 12 kinds of cells were treated with SC and ESG compounds with different concentration gradients (1-10 μM). After 44 hours of incubation, 10 μl of 3-(4,5-dimethylthiazole-2)-2,5-diphenyltetrazolium bromide (MTT) solution (5 mg / ml) was added to each well, and the incubation was continued for 4 hours. After the reaction was terminated, the culture medium was sucked off, 150 μl dimethyl sulfoxide was added to each well, shaken at a low speed to fully dissolve, and then the absorbance value at 490 nm (OD490) was measured with a microplate reader. According to the results of growth inhibition rate, the half inhibitory concentration (IC) of SC and ESG to 12 kinds of cells was calculated. 50 ), specif...
Embodiment 3
[0044] Ethoxysanguinarine or sanguinarine citrate and their pharmaceutically acceptable salts or solvates can be prepared into injections after adding water for injection according to conventional methods, finely filtering, potting and sterilizing.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com