Isothiazolinone composition
A technology of isothiazolinone and composition, which is applied in the field of isothiazolinone composition, can solve the problems of biological activity reduction, incompatibility, and precipitation, and achieve excellent stability, growth inhibition, and wide application range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
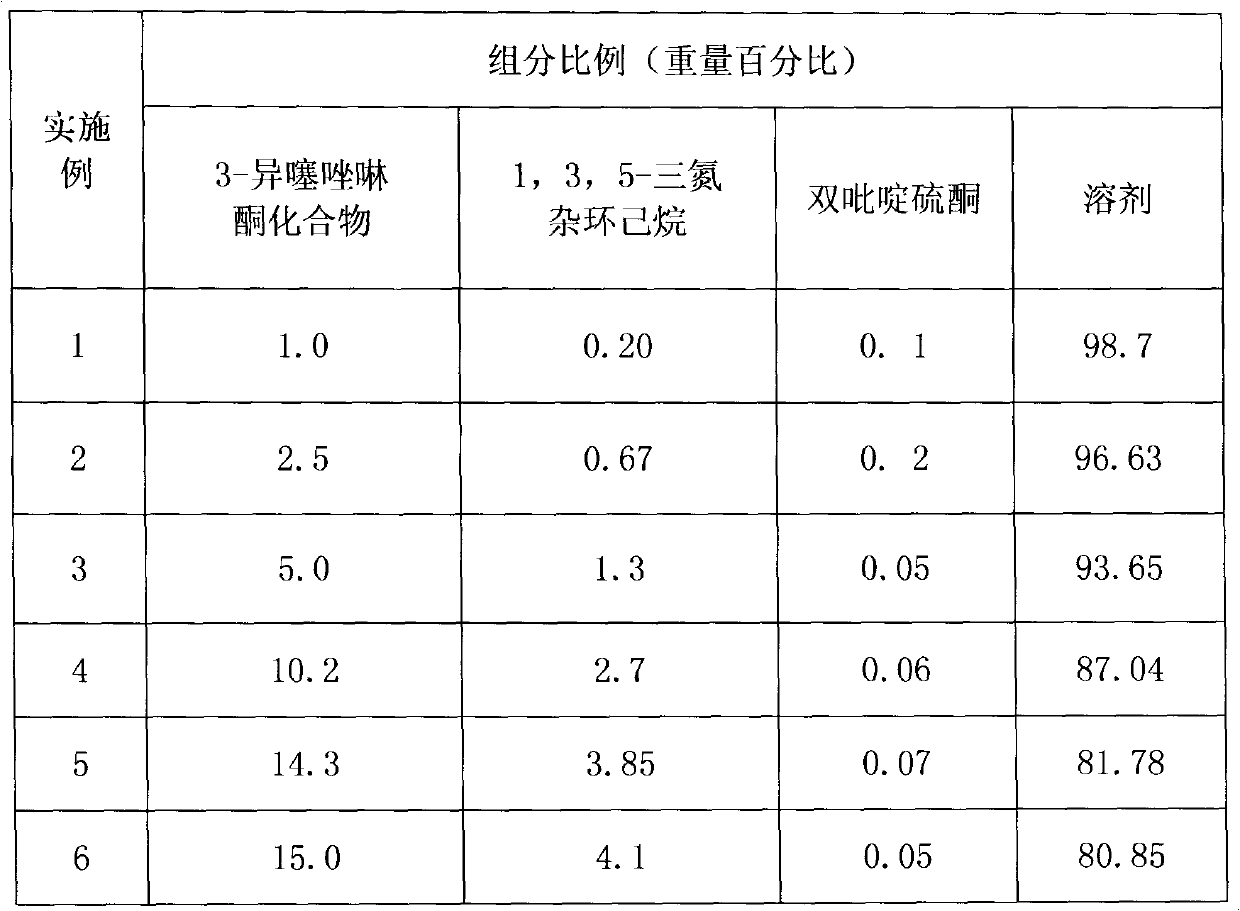
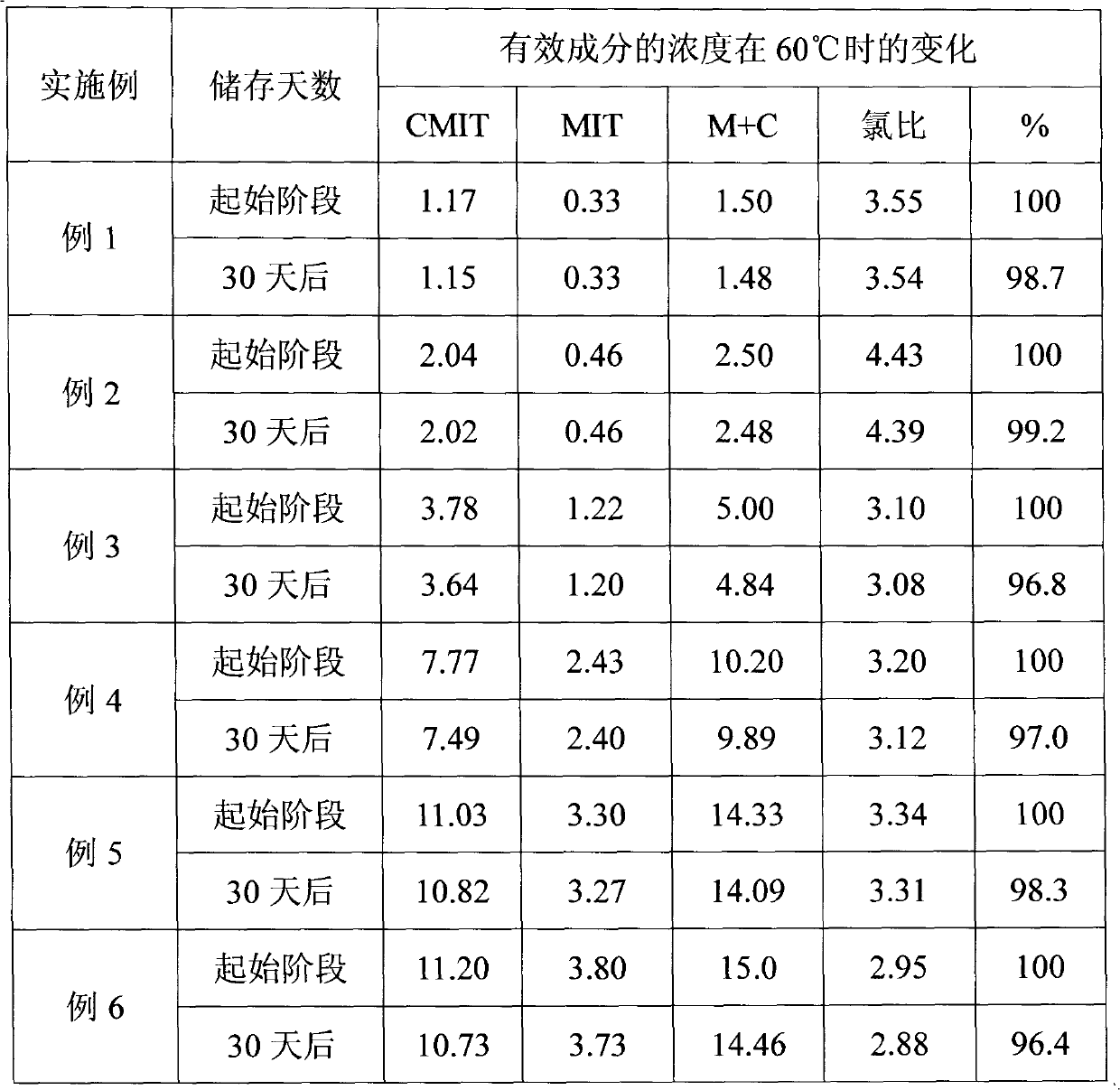
[0012] The preparation process of the 3-isothiazolinone composition of the present invention is: neutralize the 3-isothiazolinone compound in water with 1,3,5-triazacyclohexane, the pH value is 2.0-3.0, add Bipyridinethione, stirred evenly to obtain 3-isothiazolinone composition product.
[0013] The composition and content of the formulated 3-isothiazolinone compositions are shown in the table.
[0014]
[0015] The 3-isothiazolinone compound in the component can be: 5-chloro-2-methyl-3-isothiazolinone or 2-methyl-3-isothiazolinone or 5-chloro-2-ethyl -3-isothiazolinone or 2-ethyl-3-isothiazolinone or 5-chloro-2-isopropyl-3-isothiazolinone or 2-isopropyl-3-isothiazolinone or 4,5-Dichloro-2-methyl-3-isothiazolinone. It is not limited to these ingredients.
[0016] The 3-isothiazolinone compound in the component can also be a mixture of 5-chloro-2-methyl-3-isothiazolinone and 2-methyl-3-isothiazolinone, and the ratio between the two The (weight ratio) is 1:100 to 100:1, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com