Preparation method for culture medium product for medicine industry environment microorganism monitoring
A technology of environmental microorganisms and the pharmaceutical industry, applied in the field of preparation of culture medium products, can solve problems such as low work efficiency, microbial contamination, and long time required for use, and achieve the effects of improving production efficiency, simple operation, and easy control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0014] The present invention will be explained in detail below in conjunction with the experimental scheme.
[0015] 1 material
[0016] 1.1 Sterile petri dishes: provided by Qingdao Jindian Biochemical Equipment Co., Ltd., divided into: (1) Petri dishes for the determination of planktonic and sedimentary bacteria, with a size of 90×15mm; (2) Petri dishes for the determination of surface microorganisms (contact plates), Specification 65×15mm (with counting grid). The Petri dishes were sterilized by ethylene oxide to meet the requirements of sterile state.
[0017] 1.2 Soybean casein agar medium: dry medium provided by Qingdao Haibo Biotechnology Co., Ltd.
[0018] 1.3 High-density polyethylene plastic bag: Hangzhou Zijiang Plastic Products Co., Ltd.
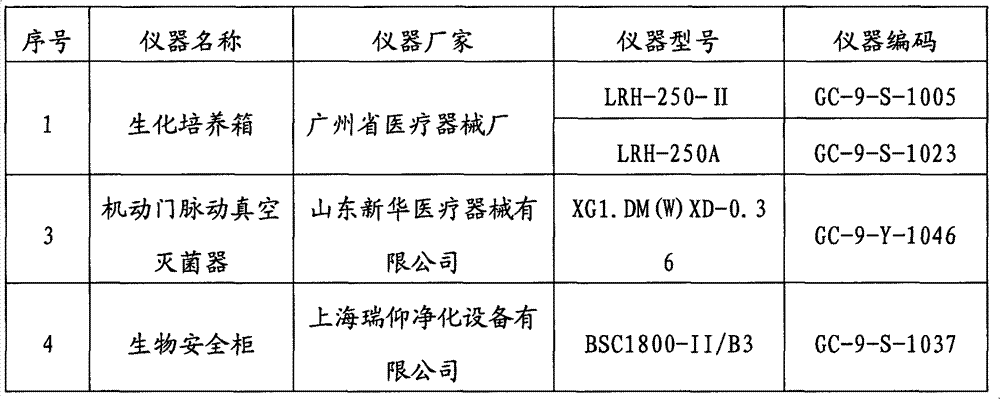
[0019] 2 instruments
[0020]
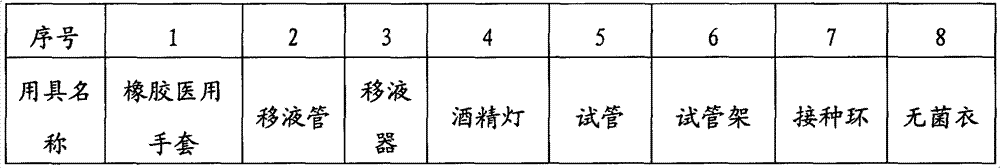
[0021] 3 utensils
[0022]
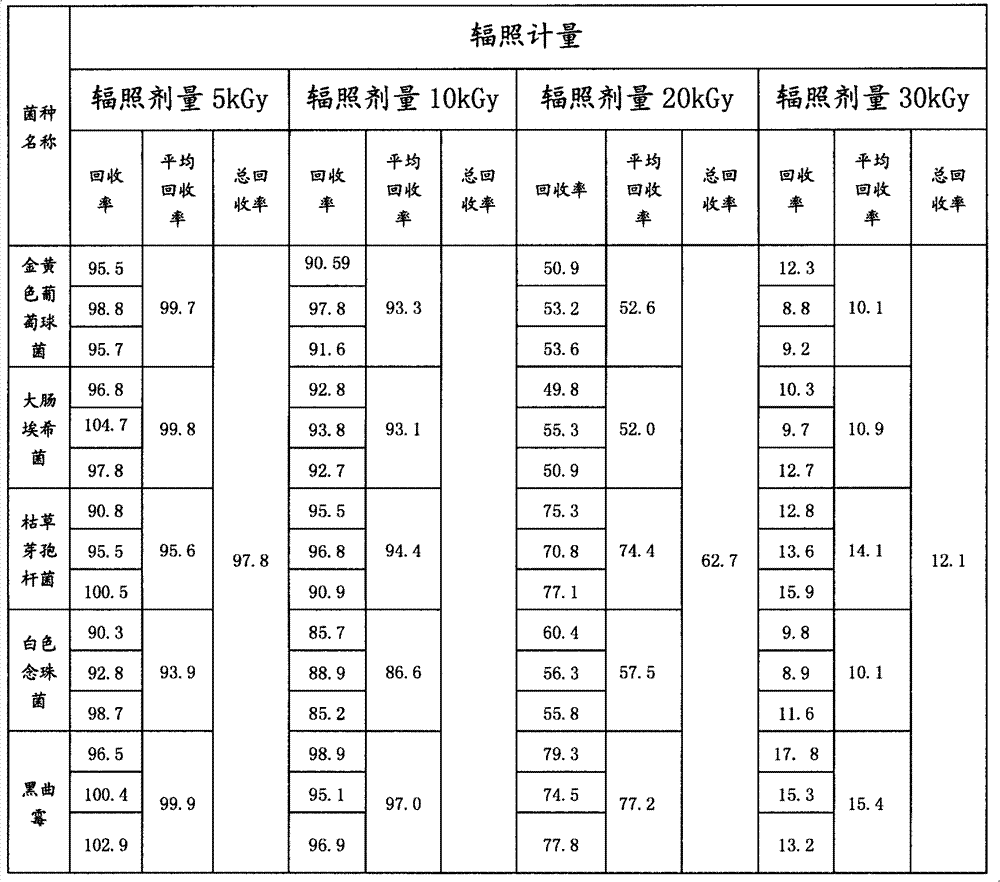
[0023] 4 strains
[0024] Source of bacteria: Zhejiang Food and Drug Inspection Institute
[0025] strain name
latin name
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com