Fluorescence quantitative polymerase chain reaction (PCR) detection kit for hepatitis B virus (HBV)
A hepatitis B virus detection kit technology, applied in the field of molecular biology, can solve the problems of difficult standardization and direct quantification of detection conditions, and achieve the effect of increasing TM value, shortening length and high resolution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
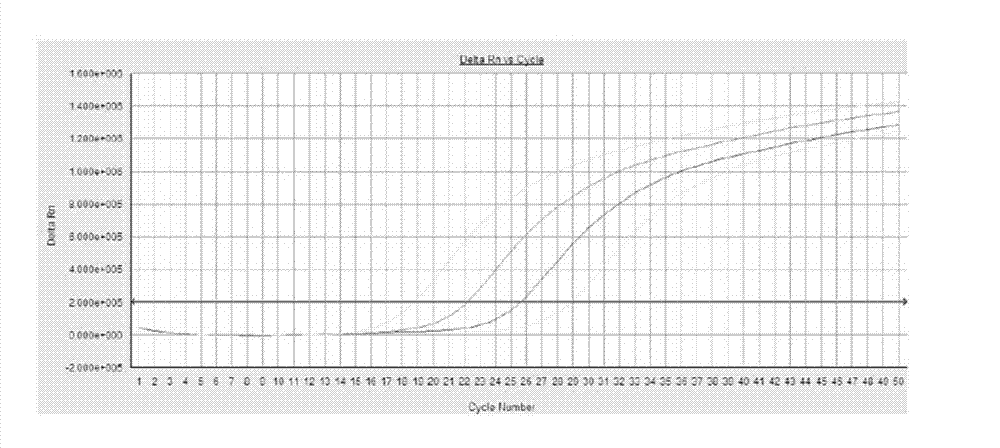
[0034] Embodiment 1: the sensitivity of the kit of the present invention for standard samples
[0035] The detection concentration of the fluorescent quantitative PCR detection kit of the present invention is respectively 10 4 copies / µl, 10 5 copies / µl, 10 6 copies / µl, 10 7 copies / μl of HBV DNA standard. The standard product in this experiment is the plasmid DNA synthesized by Treasure Bioengineering (Dalian) Co., Ltd.
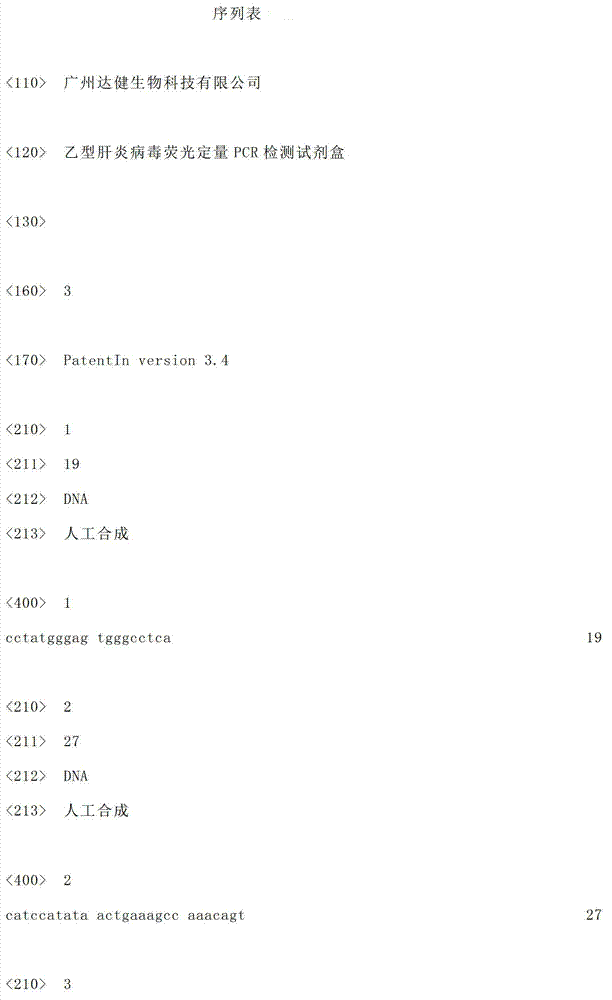
[0036] The kit of the present invention contains a specially designed fluorescent probe and a pair of primers that can specifically detect HBV DNA:
[0037] Forward primer (HBV-Forward Primer):
[0038] 5'-CCTATGGGAGTGGGCCTCA-3'
[0039] Reverse primer (HBV-Reverse Primer):
[0040] 5'-CATCCATATAACTGAAAGCCAAACAGT-3'
[0041] Fluorescent probe (HBV-Taqman-Probe):
[0042] 5'-FAM-CTAGTGCCATTTGTTC-MGB-3'
[0043] Then optimize the reaction system for FQ-PCR detection, the fluorescence quantitative reaction is carried out on the ABI7900 detector, the rea...
Embodiment 2
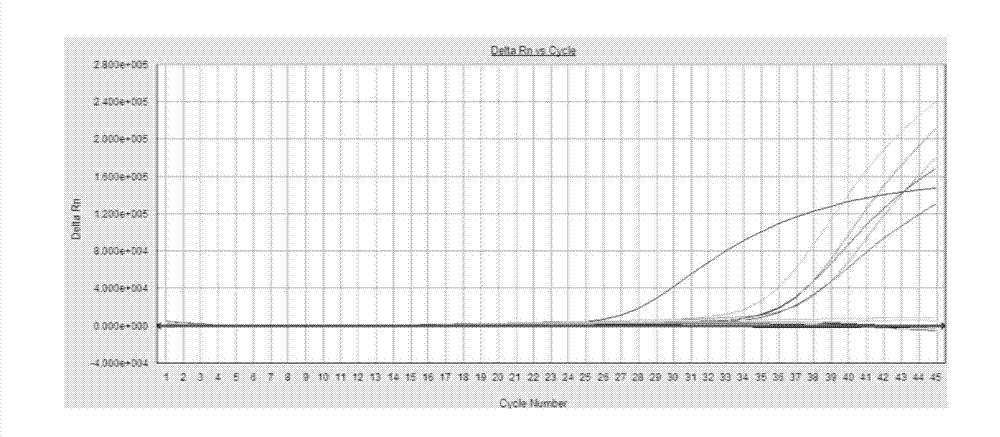
[0047] Example 2: Sensitivity of the kit of the present invention for clinical samples
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com