Application of endothelium corneum gigeriae galli and salviae miltiorrhizae capsules for preparing medicines for relieving asthma
A technology of Jinshen Shugan capsule and anti-asthma, applied in the directions of capsule delivery, drug combination, pharmaceutical formula, etc., can solve the problem of untreated asthma, etc., and achieve good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
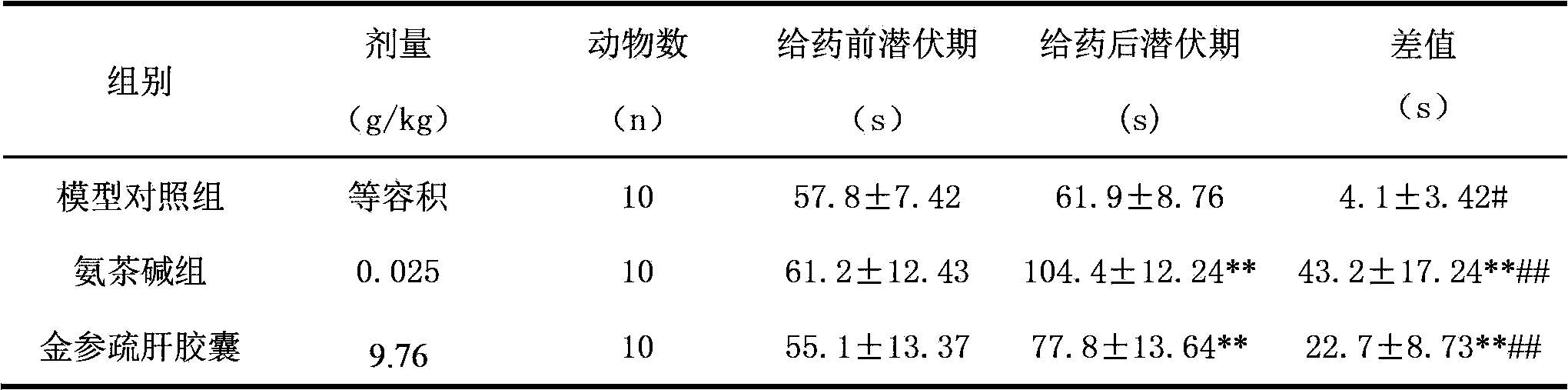
[0008] Experimental Research Data of Jinshen Shugan Capsules in Treating Asthma
[0009] 1. Research drug: Jinshen Shugan Capsule, Harbin Shengtai Pharmaceutical Co., Ltd., batch number: 20110424
[0010] 2. Animals: guinea pigs, half male and half male, weighing about 160-200g, provided by Qinglongshan Animal Breeding Farm, Tangshan, Jiangning County, experimental animal production license SCXK (Su) 2002-0004, experimental animal use license: SYXK (Su) 2002 -0008.
[0011] 3. Reagents: acetylcholine chloride (Shanghai Sanaisi Reagent Co., Ltd., batch number: 20080527), histamine phosphate (sigma, batch number: 31K2615).
[0012] 4. Method: The day before the experiment, the guinea pigs were screened in advance, and the guinea pigs were sprayed with an equal volume mixture of 2% acetylcholine chloride and 0.1% phosphate histamine for 15 seconds, and the incubation period of the guinea pigs was observed (that is, from the beginning of spraying to the onset of asthma, breathing...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com