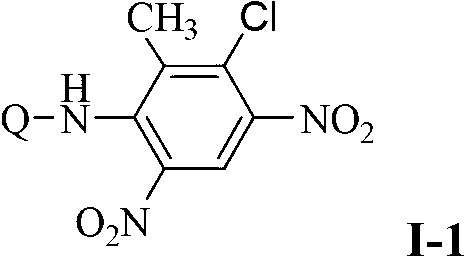
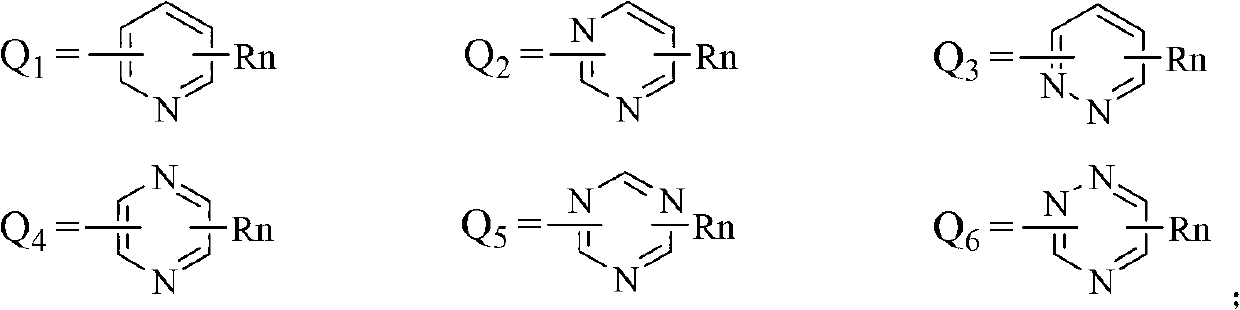
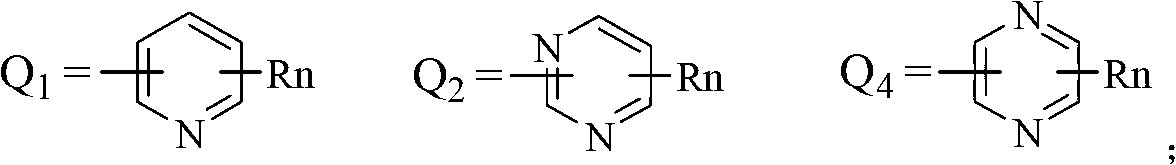
Substituted nitroaniline compound and its application
A technology of nitroaniline and compounds, which is applied in the field of substituted nitroaniline compounds, and can solve problems such as unreported structural compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0126] Embodiment 1: the preparation of compound IA-1
[0127]
[0128] Dissolve 0.47g (0.005mol) of 2-aminopyridine in 10mL of N,N-methylformamide, add 0.4g (0.01mol) of sodium hydroxide under stirring, and add 1.26g (0.005mol) to it after 10 minutes After adding 2,6-dichloro-3,5-dinitrotoluene, continue stirring at room temperature for 5 hours. After TLC monitors that the reaction is complete, the reaction solution is poured into 50 mL of saturated brine, extracted with ethyl acetate, the organic phase is dried with anhydrous magnesium sulfate and precipitated under reduced pressure, and the residue is column chromatographed (eluent is ethyl acetate and petroleum ether). (boiling range 60-90°C), volume ratio 1:10) purified to obtain 1.21 g of solid, namely compound IA-1. The melting point is 111-113°C.
[0129] 1 H-NMR (300MHz, internal standard TMS, solvent CDCl 3 )δ(ppm):2.26(s,3H),6.90(d,1H),6.96-7.00(m,1H),7.65-7.71(m,1H),8.19-8.22(m,1H),8.60(s ,1H), 8.94(d,1H). ...
Embodiment 2
[0130] Embodiment 2: the preparation of compound IA-28
[0131]
[0132] Dissolve 0.64g (0.005mol) of 2-amino-5-chloropyridine in 10mL of N,N-dimethylformamide, add 0.4g (0.01mol) of sodium hydroxide under stirring, and add 1.26 g (0.005mol) 2,6-dichloro-3,5-dinitrotoluene, after the addition, continue to stir at room temperature for 5 hours. After TLC monitors that the reaction is complete, the reaction solution is poured into 50 mL of saturated brine, extracted with ethyl acetate, the organic phase is dried with anhydrous magnesium sulfate and precipitated under reduced pressure, and the residue is column chromatographed (eluent is ethyl acetate and petroleum ether). (Boiling range 60-90°C, volume ratio 1:10) Purified to obtain 1.45 g of solid, namely compound IA-28. The melting point is 168-170°C.
[0133]1 H-NMR (300MHz, internal standard TMS, solvent CDCl 3 )δ (ppm): 2.25 (s, 3H), 6.86 (d, 1H), 7.63 (dd, 1H), 8.13 (d, 1H), 8.59 (s, 1H), 8.89 (s, 1H).
Embodiment 3
[0134] Embodiment 3: the preparation of compound IA-230
[0135]
[0136] Dissolve 1.16g (0.005mol) of 3-amino-2,6-dichloro-4-trifluoromethylpyridine in 10mL of N,N-dimethylformamide, and add 0.4g (0.01mol) of hydrogen under stirring Sodium oxide, 1.26g (0.005mol) 2,6-dichloro-3,5-dinitrotoluene was added to it after 10 minutes, and stirring at room temperature was continued for 5 hours after the addition. After TLC monitors that the reaction is complete, the reaction solution is poured into 50 mL of saturated brine, extracted with ethyl acetate, the organic phase is dried with anhydrous magnesium sulfate and precipitated under reduced pressure, and the residue is column chromatographed (eluent is ethyl acetate and petroleum ether). (Boiling range 60-90°C, volume ratio 1:10) Purified to obtain 1.88g of compound IA-230. The melting point is 130-132°C.
[0137] 1 H-NMR (300MHz, internal standard TMS, solvent CDCl 3 )δ (ppm): 2.00 (s, 3H), 7.67 (s, 1H), 8.74 (s, 1H), 9.59 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com