Polyhalite IMI process for KNO3 production
A kind of polyhalite, NO3- technology, applied in the field of preparing KNO3
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
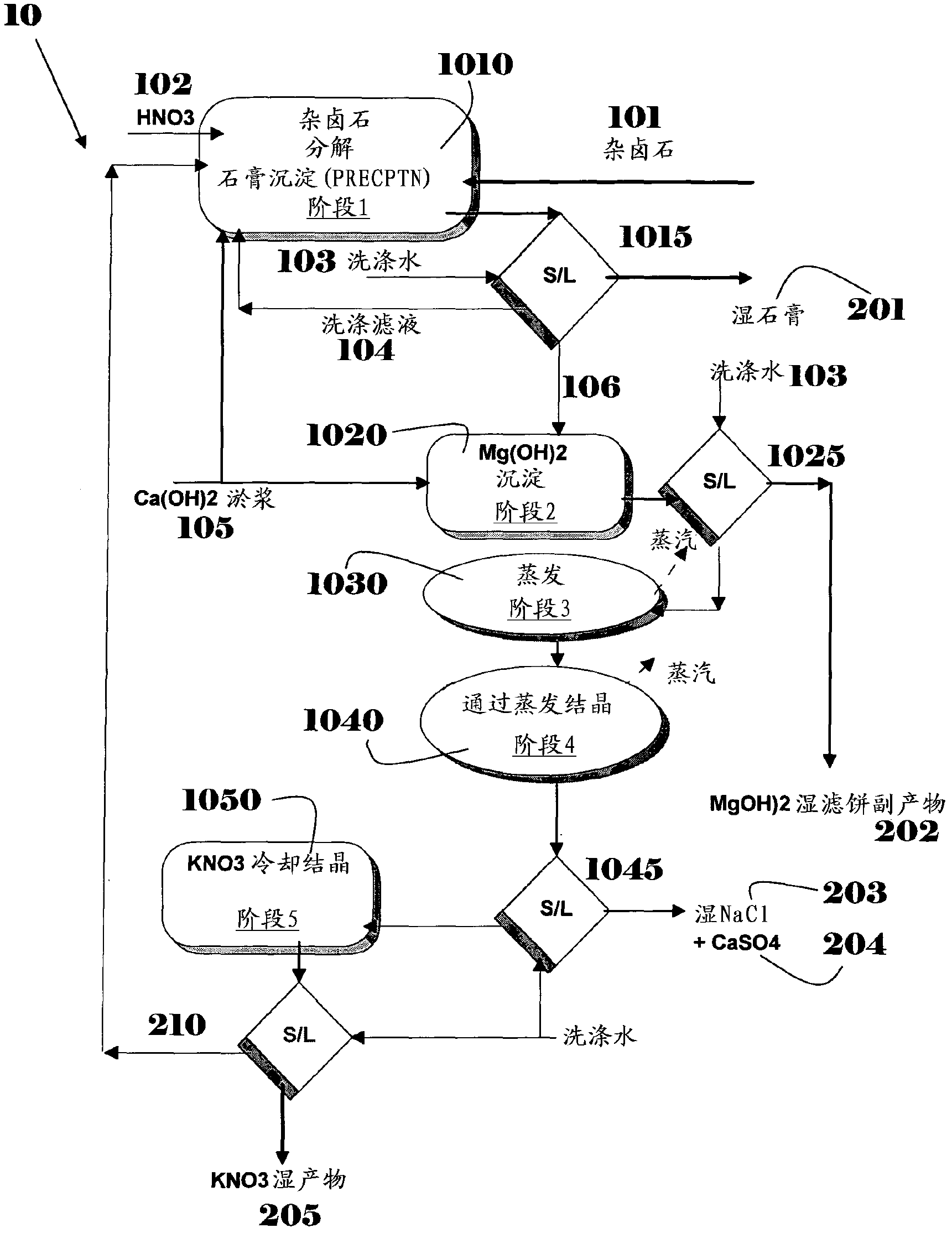
[0064] To stirred nitric acid (59%, 146.7g) and recirculation solution (1090g, combined from the KNO given in Example 3 3 Polyhalite (unwashed, crushed and sieved to ~0.5 mm, 400 g) was added to a mixture of crystallized mother liquor and gypsum wash water from a previous batch). The concentration of nitric acid was varied by diluting with wash water from previous runs to maintain a constant nitrate concentration of 15-16% in the final filtrate. The reaction mixture was heated to 65 °C and stirred for 3 h. Afterwards, milk of lime (169.4 g, 30% strength in water) was added dropwise via pump to the hot mixture during 1 hour to neutralize the acidity of the slurry (slurry). When the mixture reached a pH of 5.5-6.5, the addition was stopped and the mixture was filtered under vacuum while still hot. The gypsum filter cake (700 g, 60.8% solids) was then washed with water (3 x 350 g) so that the nitrate content of the filter cake was satisfactorily low. The wet washed gypsum (575...
Embodiment 2
[0066] A sample of the solution obtained after completion of the reaction given in Example 1 above (720-900 g of solution) was treated with a 15% milk of lime solution (300 g) at a temperature of 60-70°C. As a result of this treatment, the Mg concentration was reduced from 1.5% to below 0.2%. The precipitated solid was allowed to settle, then filtered and washed. Dry solids contain more than 92% Mg(OH) 2 . The main impurities are Ca (4 2- (2%), NO 3 - (0.2%) and Cl - (0.05%).
Embodiment 3
[0068] Concentrate the precipitated Mg(OH) as described in Example 2 above by evaporation at a temperature in excess of 80° C. 2 A sample of the remaining solution containing (concentrations in w / w relative to the total solution) 2.2% Ca, 4.4% K, 1.9% Na, 0.01% Mg, 13.3% NO 3 - , 3.1% Cl - and 0.08% SO 4 2- . As a result of concentration, the total concentration of dissolved salts increased by >80%. The NaCl thus crystallized is isolated at a temperature of more than 80 °C and its purity exceeds 98% after washing.
[0069] The remaining solution is then cooled to a temperature 3 Precipitated, then separated from mother liquor and washed. Income KNO 3 The purity of is more than 99.5%, while the concentration of dissolved salts in the mother liquor to be recycled back to the reaction is in the range of 55-60%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com