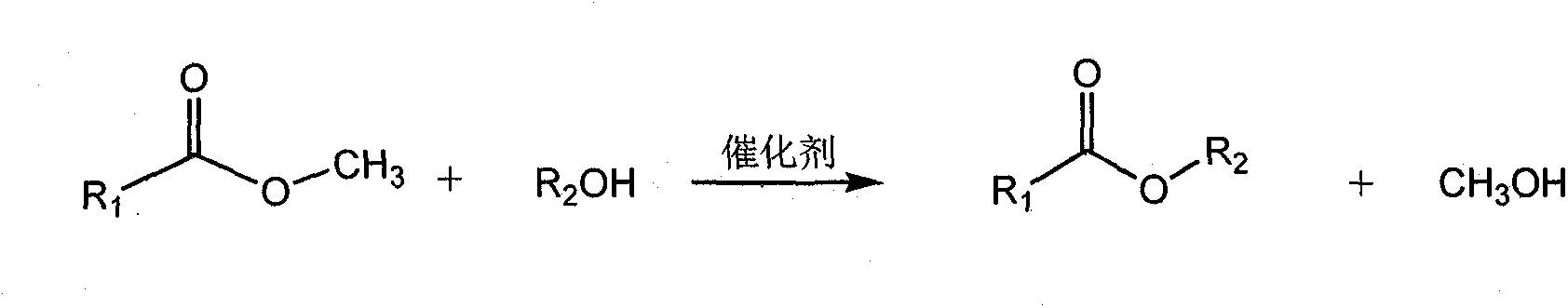
Method for catalyzing ester exchange reaction with alkaline ionic liquid
A basic ion exchange reaction technology, applied in chemical instruments and methods, physical/chemical process catalysts, organic compounds/hydrides/coordination complex catalysts, etc., can solve the problem of harsh process conditions and increase equipment investment in transesterification reactions and difficulty in operation, insufficient stability of catalyst heating, etc., to achieve the effects of large-scale industrial production, reduced equipment investment, and excellent thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Quaternization reaction: In a 100mL round bottom flask, add 0.1mol (13.9g) 1,5,7-triazide bicyclo (4.4.0) dec-5-ene, 0.1mol (13.7g) n-butyl bromide Alkanes and 30mL cyclohexane, heated to reflux, stirred for 2 to 3 hours, cooled, filtered, washed with cyclohexane to remove unreacted raw materials, dried in vacuo to obtain a quaternized intermediate with a yield of 90%;
[0023] Ion exchange reaction: In a 50mL round bottom flask, add 0.05mol (2.81g) of potassium hydroxide, 0.05mol (13.8g) of the above-mentioned quaternized intermediate, and 10mL of absolute ethanol, and stir the reaction at room temperature 5-6 hour, filtered, and evaporated ethanol to obtain the target compound ionic liquid, the yield was 85%, and the spectral characterization data were as follows:
[0024] 1 H-NMR (500MHz, DMSO-d 6 , ppm): 0.97(t, J=7Hz, 3H), 1.34-1.40(m, 2H), 1.55-1.60(m, 2H), 1.96-2.05(m, 4H), 3.27-3.32(m, 4H) , 3.33-3.43(m, 8H); 13 C-NMR (125MHz, DMSO-d 6 , ppm): 14.4, 21.0, 2...
Embodiment 2
[0026] In a 100mL round bottom flask, add 0.067mol (20g) of fatty acid methyl ester, 0.067mol (4.97g) of n-butanol and 0.1g of multi-site basic ionic liquid, heat to 100°C, mix and stir under normal pressure for 5.0 Hours, cooled to room temperature, transferred to a separatory funnel and allowed to stand for stratification. The lower layer was a mixture of ionic liquid catalyst, methanol, and unreacted butanol. The methanol was removed by 70°C atmospheric distillation and 70°C vacuum distillation at 1.0kPa. and butanol, the remainder is an ionic liquid catalyst, which can be recycled and reused; the upper layer is the crude product of fatty acid n-butyl ester, washed with water, dried, and can obtain a refined product by distillation under reduced pressure at 400Pa or rectification with a rectifying tower, and the yield is 85%.
Embodiment 3
[0028] In a 100mL round bottom flask, add 0.067mol (20g) of fatty acid methyl ester, 0.067mol (4.97g) of n-butanol and 1.0g of multi-site basic ionic liquid, heat to 65°C, mix and stir under normal pressure for 6.0 hour, cooled to room temperature, all the other operations were the same as in Example 2, and the productive rate was 82%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com