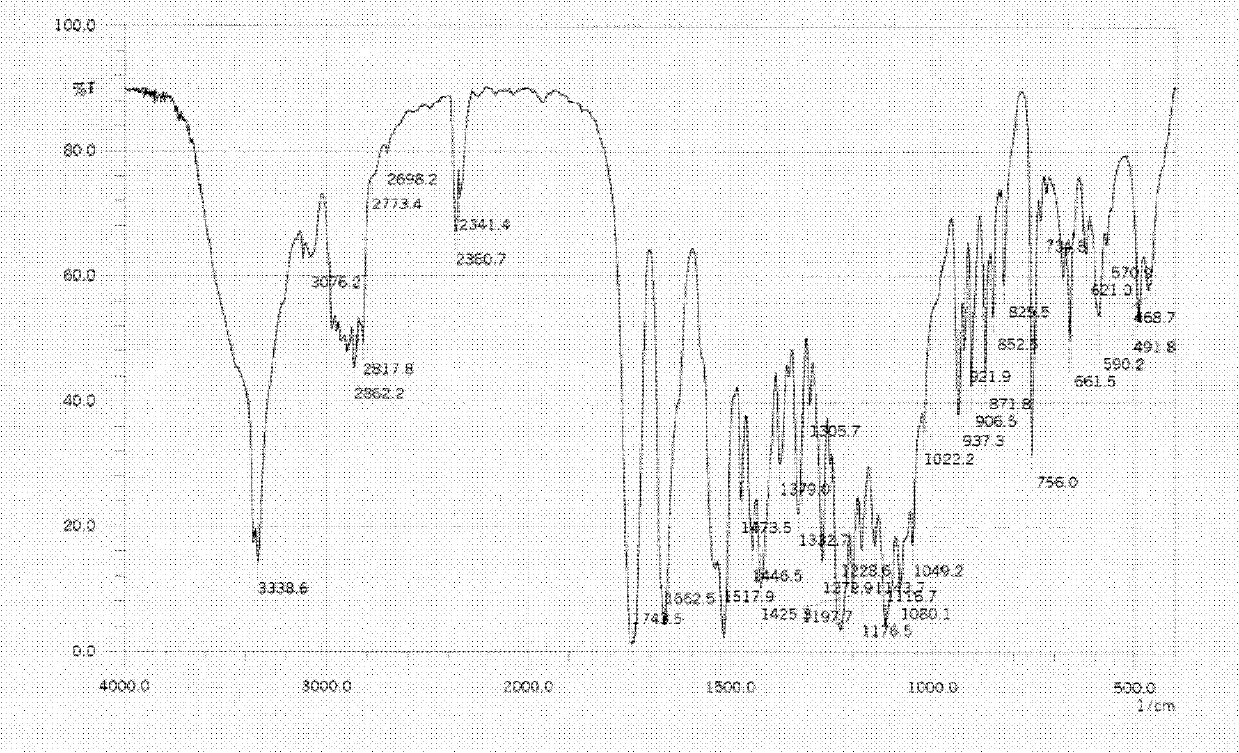
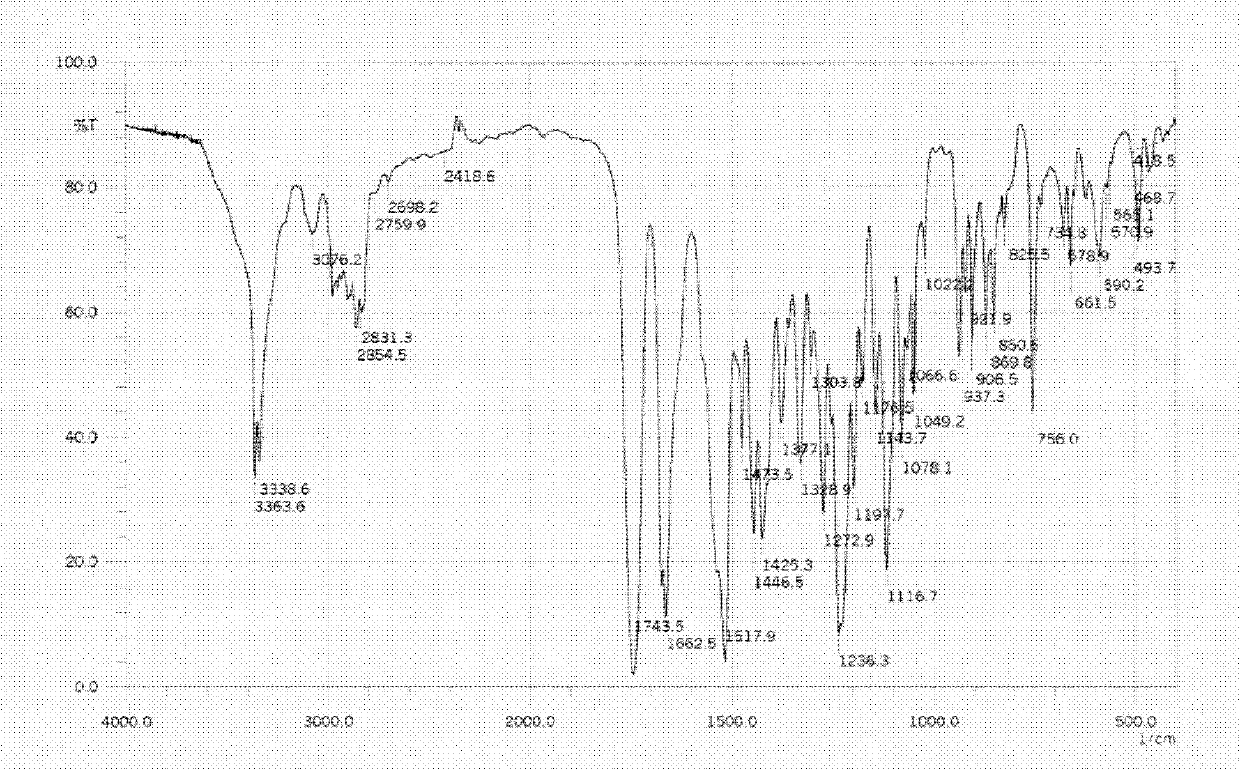
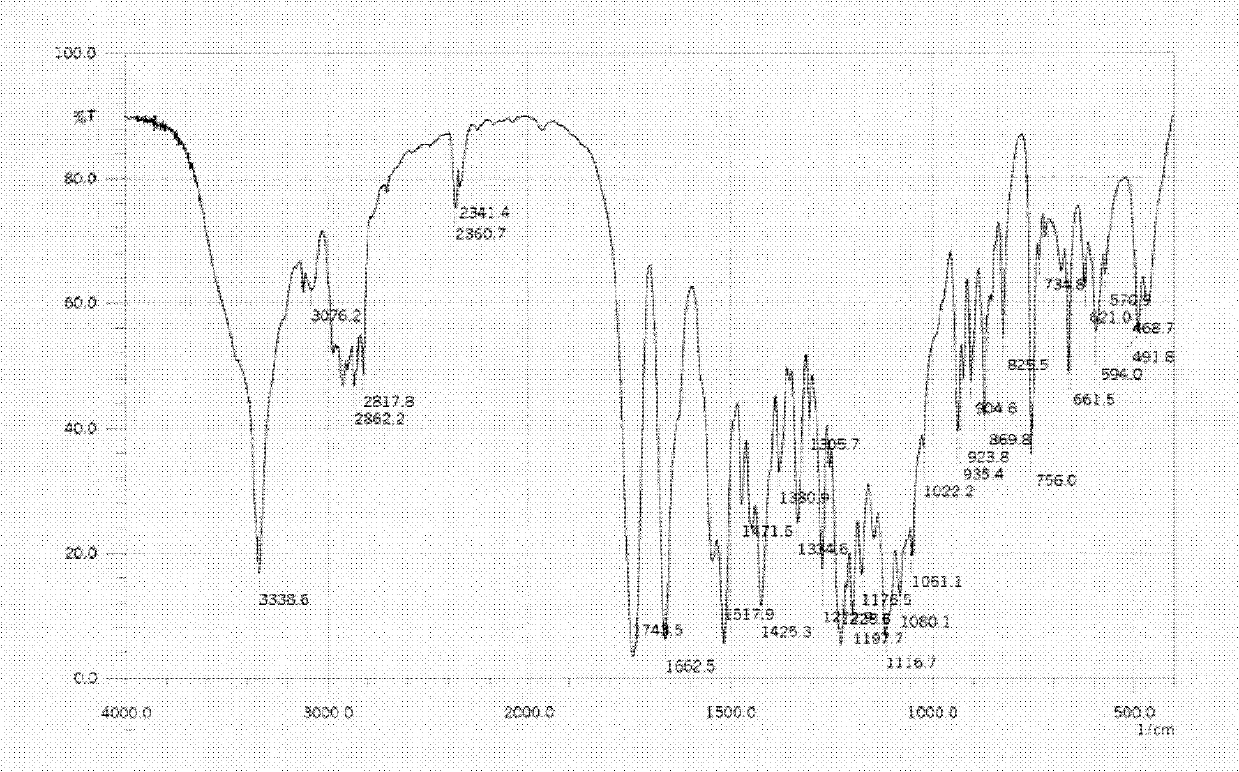
Linezolid tablets in stable crystal form and preparation method thereof
A technology of linezolid tablets and linezolid, which is applied in the field of linezolid tablets and its preparation, can solve problems such as cracks, unstable crystal form of linezolid tablets, unstable crystal form of linezolid, etc., and achieve dissolution rate Good, good compressibility effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1 Preparation of linezolid tablets (adding auxiliary materials after granulation)
[0037] Take 300g of linezolid crystal form IV, add 18g of the melted solution of polyoxy(40) stearate, mix uniformly to prepare granules, the prepared granules are added with 284g of microcrystalline cellulose, 174g of dextrin, 80g of low-substituted carboxylate Propyl cellulose and 4 g of magnesium stearate are mixed uniformly and compressed to obtain 1000 linezolid tablets.
[0038] The preparation methods of Examples 2 to 8 are the same as in Example 1, and the types and amounts of raw materials used are shown in Table 1. 1000 linezolid tablets were prepared respectively.
Embodiment 9
[0039] Example 9 Preparation of linezolid tablets (adding auxiliary materials before granulation)
[0040] Take 700g of linezolid crystalline form IV, mix it with 92g starch and 24g microcrystalline cellulose, add 72g polyethylene glycol 6000 melt, mix to make granules, the prepared granules add 18g talc powder and 4g stearic acid Magnesium was mixed well and compressed to obtain 1000 linezolid tablets.
Embodiment 10
[0041] Example 10 Preparation of linezolid tablets (adding auxiliary materials before granulation)
[0042] Take 340g of linezolid crystal form IV, mix it with 39g of talc, 4g of magnesium stearate, 155g of lactose, and 22g of sodium carboxymethyl starch, then add 240g of cetyl alcohol melt, mix to make granules, press to obtain 1000 linezolid tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com