Anti-cd160 specific antibodies for the treatment of eye disorders based on neoangiogenesis
A-CD160, eye disease technology, applied in cardiovascular system diseases, antibodies, metabolic diseases, etc., can solve problems such as increasing the risk of harmful events
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
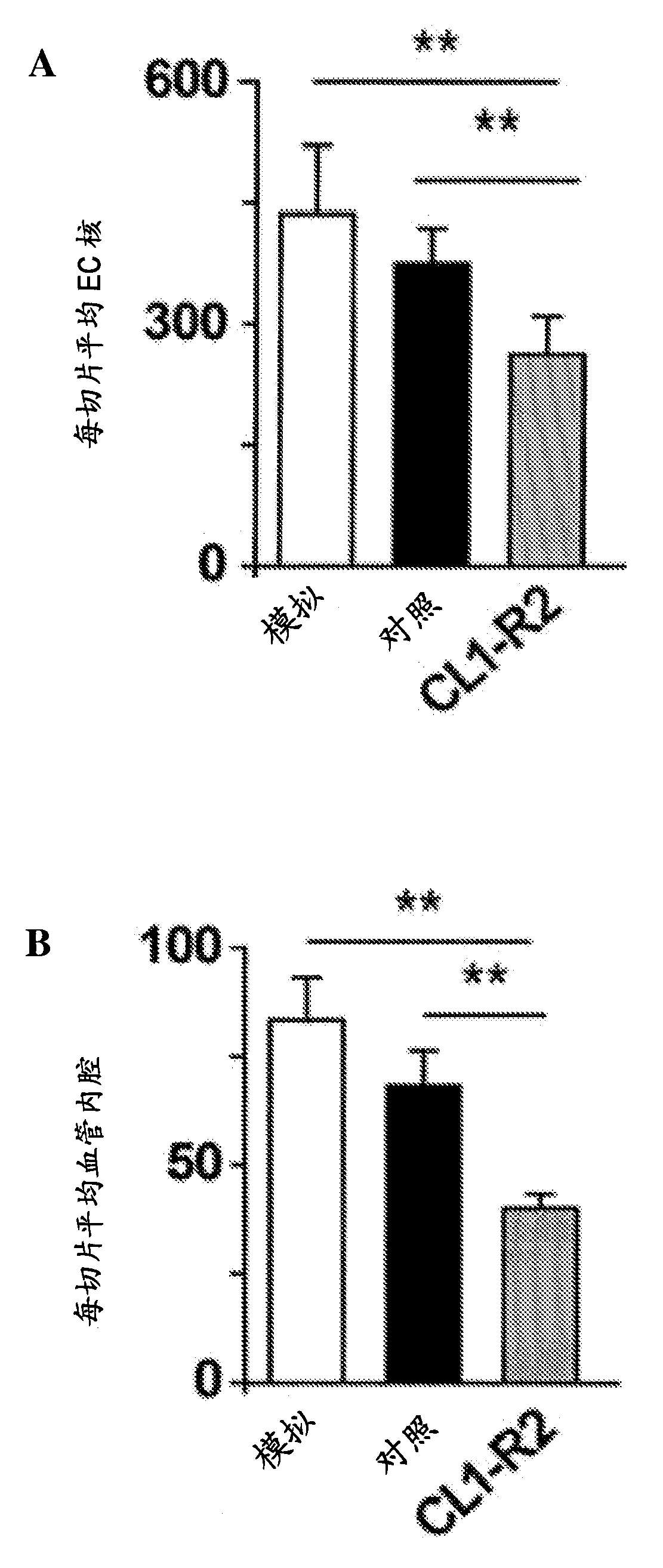
Embodiment 1
[0120] Materials and methods
[0121] Murine anti-CD160 CL1-R2 mAb. The mouse anti-CD160CL1-R2 mAb (IgG1) was grown in our laboratory and evaluated as an anti-CD160 mAb during the 7th Human Leukocyte Differentiation Antigen Workshop (26). We prepared CL1-R2 mAb from a specific secreting hybridoma cell line by using the high cell density system CeLLine (VALDEA Biosciences). Mouse IgGl isotype control monoclonal antibody He6 was generated by immunizing mice with hepatitis B surface antigen (HB). Both CL1-R2 and IgG1 isotype controls were passed in HiTrap in the purification system TM Affinity chromatography on a protein G column (GE Healthcare) was similarly purified, dialyzed against PBS pH 7.0, concentrated and filtered through a 0.22-μm filter.
[0122] animal. We used BALB / c, C57BL / 6J and NMRI-nu (nu / nu) nude mice (Janvier Laboratories). Mice were 7-10 weeks old, except for those used for ischemic retinopathy experiments, which were 7 days old. Animals were housed in...
Embodiment 2
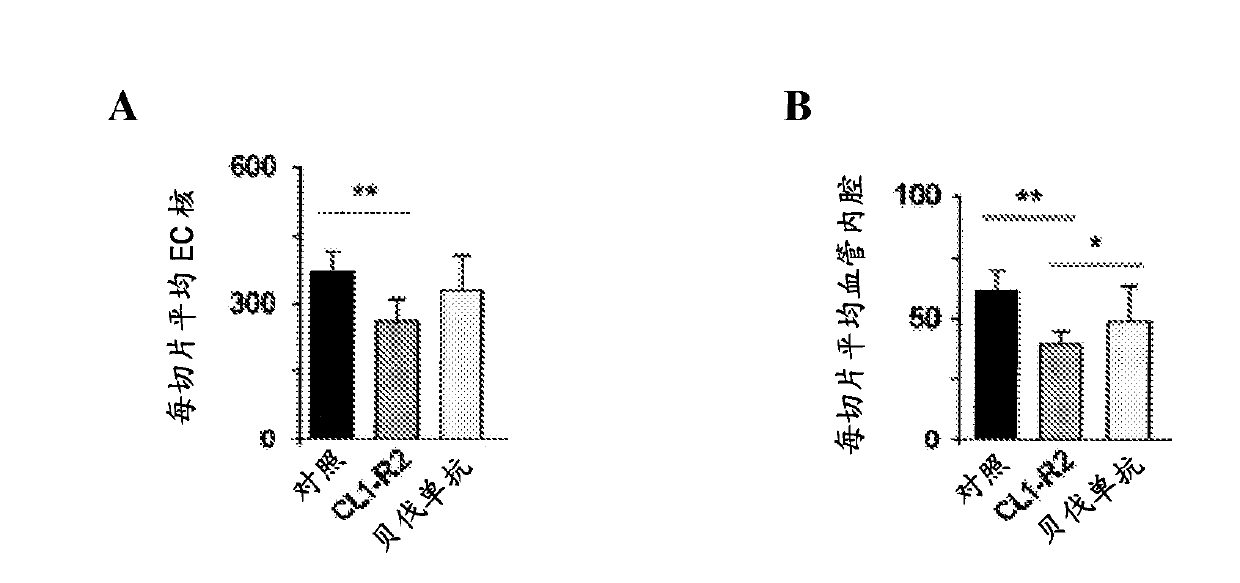
[0130] Example 2: Synergistic effect of CL1R2 and anti-VEGF antibodies on inhibition of neovascularization
[0131] By using the corneal angiogenesis model with FGF2-treated implants as in Example 1, the inventors evaluated the CL1-R2 mAb in combination with an anti-VEGF antibody (Avastin ) in vivo anti-angiogenic properties.
[0132] For this purpose, they compared the results obtained from several groups of rabbits administered with:
[0133] - IgG1 only (25 μg injected);
[0134] -Avastin only (25 μg injection 2 times);
[0135] - CL1-R2 only (2 injections of 25, 50 or 100 μg);
[0136] -Avastin in combination with IgG1; and
[0137] -Avastin Combined with CL1-R2.
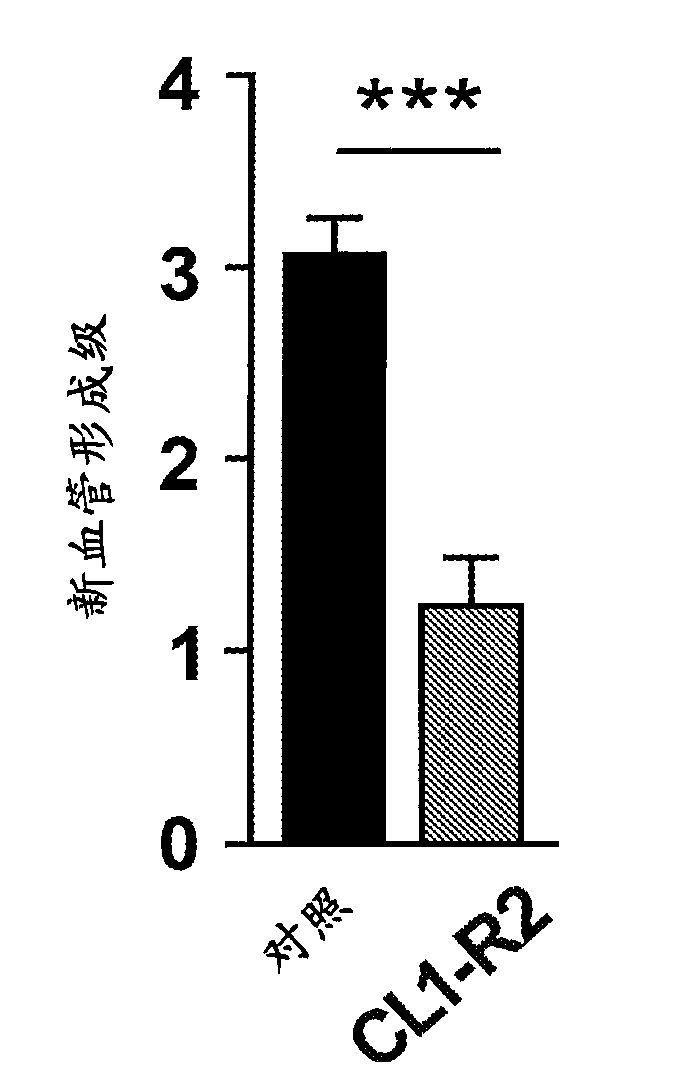
[0138] Each group consisted of 4 rabbits. The grade corresponds to the length of the neovascularization.
[0139] The results are shown in Figure 4 . The inventors demonstrate that using CL1-R2 and anti-VEGF antibodies together provides better results in inhibiting neovascularization in an ocu...
Embodiment 3
[0141] Example 3: Effect of intact and Fab'2 forms of CL1-R2
[0142] The inventors compared the effect of the intact form of CL1-R2 and the Fab'2 form of CL1-R2 on corneal neovascularization. For this purpose they used a control IgG1.
[0143] The results are disclosed in Figure 5. The grade corresponds to the length of the neovascularization. The inventors have shown that the intact form of CL1-R2 provides better results in inhibiting neovascularization than that provided by control IgGl.
[0144] They further demonstrated that the Fab'2 format is very suitable for inhibiting neovascularization as it provided better results compared to the intact form CL1-R2 and control IgG1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com