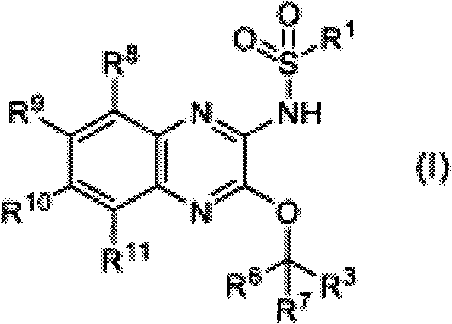
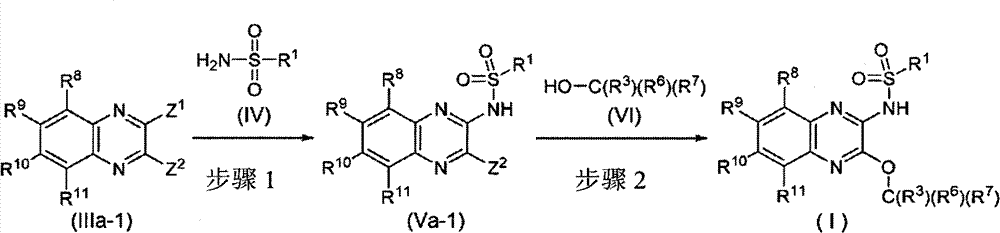
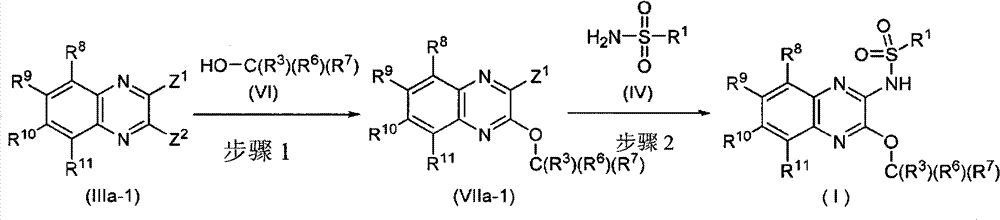
Nitrogen-containing heterocyclic compound having kynurenine production inhibitory activity
A technology of nitrogen heterocyclic compounds and kynurenine, which is applied in the field of kynurenine production inhibitors and can solve problems such as tumor growth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
reference example 1
[0249] N-(3-Chloroquinoxalin-2-yl)propane-1-sulfonamide (Compound A1)
[0250] Dissolve 2,3-dichloroquinoxaline (5.00g, 25.1mmol) and propane-1-sulfonamide (3.09g, 25.1mmol) in DMSO, add potassium carbonate (3.47g, 25.1mmol), and stir at 150°C 1 hour. Add 1% aqueous acetic acid solution to the reaction mixture, stir at room temperature for 3 hours, filter the resulting solid, wash with water, add diisopropyl ether, and refine the slurry to obtain compound A1 (6.01g, yield 84%) .
reference example 2
[0252] N-(3-Chloroquinoxalin-2-yl)butane-1-sulfonamide (compound A2)
[0253] According to Reference Example 1, compound A2 was obtained from 2,3-dichloroquinoxaline and butane-1-sulfonamide.
reference example 3
[0255] N-(3-Chloroquinoxalin-2-yl)-2-methylpropane-1-sulfonamide (compound A3)
[0256] According to Reference Example 1, compound A3 was obtained from 2,3-dichloroquinoxaline and 2-methylpropane-1-sulfonamide.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com