Preparation method of oxalic acid diisoamyl ester
A technology of diisoamyl oxalate and oxalic acid, which is applied in the field of ester preparation, can solve problems such as poor thermal stability, inapplicability to higher temperature esterification systems, and use restrictions, and achieve the effect of improving conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
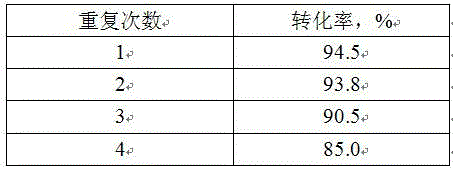
Image
Examples
Embodiment 1
[0011] Add 2.5 moles of isoamyl alcohol and 1 mole of oxalic acid into the four-neck flask, mix well and then heat slowly while stirring. After the reaction liquid reaches the specified temperature, add 2% NKC-9 catalyst, continue heating to boiling, and control the temperature of the reaction liquid 110°C for 3.0 hours, finally cooled, filtered out the catalyst, and distilled at a vacuum of 0.084MPa to collect fractions at 184-190°C, which were proved to be diisoamyl oxalate by infrared spectroscopy, gas chromatography-mass spectrometry, and the conversion rate was 93.1%.
Embodiment 2
[0013] Add 4.5 moles of isoamyl alcohol and 1 mole of oxalic acid into the four-neck flask, mix well and then heat slowly while stirring. After the reaction liquid reaches the specified temperature, add 1.5% NKC-9 catalyst, continue heating to boiling, and control the temperature of the reaction liquid 130°C for 3.5 hours, finally cooled, filtered out the catalyst, and distilled at a vacuum of 0.084MPa to collect fractions at 184-190°C, which were proved to be diisoamyl oxalate by infrared spectroscopy, gas chromatography-mass spectrometry, and the conversion rate was 94.5%.
Embodiment 3
[0015] Add 4 moles of isoamyl alcohol and 1 mole of oxalic acid into the four-necked flask, mix well and then heat slowly while stirring. After the reaction liquid reaches the specified temperature, add NKC-9 catalyst, continue heating to boiling, and control the temperature of the reaction liquid to 120°C After 3.1 hours, cool down, filter out the catalyst, and distill at a vacuum of 0.084MPa to collect fractions at 184-190°C. It is proved to be diisoamyl oxalate by infrared spectrum, gas chromatography-mass spectrometry, and its conversion rate is 94.7%. .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com