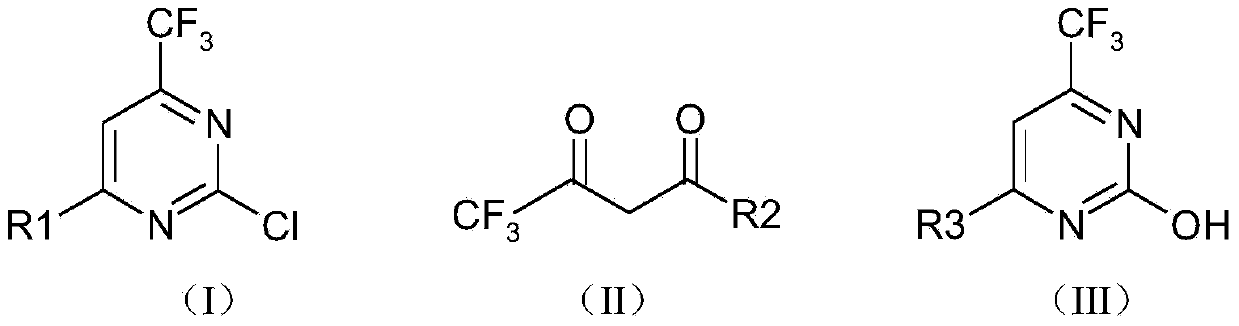
Preparation method of 2-chloro trifluoromethyl pyrimidine compound
A technology of chlorotrifluoromethylpyrimidine and trifluoromethylpyrimidine, applied in the field of organic synthesis, can solve the problems of improving yield, many by-products, low yield and the like, and achieves easy operation, low cost and high yield. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
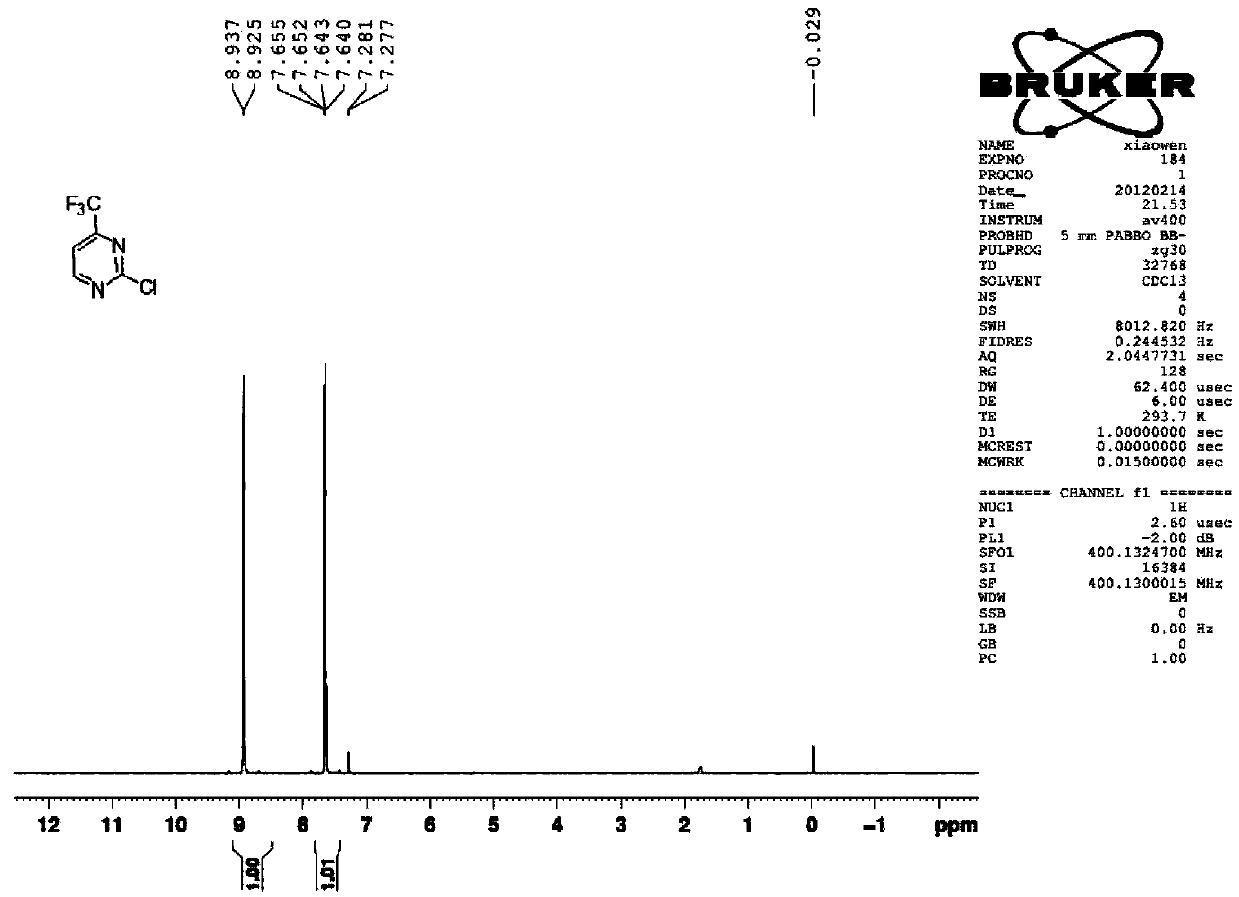
[0030] Example 1 Synthesis of 2-chloro-4-trifluoromethylpyrimidine (CAS33034-67-2)
[0031]
[0032] Trifluoroacetylacetaldehyde (14.8 grams, 0.106mol), urea (7.6 grams, 0.127mol), sodium ethylate (28.8 grams, 0.424mol) were added to the mixed solvent of 120ml toluene and 40ml ethanol, and the reaction apparatus was added Trap. Raise the reaction temperature from normal temperature to 120°C to 130°C and reflux for 12 hours. During the reaction, use a water separator to separate water from oil to remove water. The reaction solvent was spin-dried, adjusted to neutrality by adding 10% hydrochloric acid, and extracted three times with 50 ml of ethyl acetate. The ethyl acetate phases were combined, dried, filtered and spin-dried to obtain the crude product, which was washed with petroleum ether to obtain 15.7 g of 2-hydroxy-4-trifluoromethylpyrimidine, with a yield of 90.3%.
[0033] 2-Hydroxy-4-trifluoromethylpyrimidine (15.7 g, 0.096 mol) was added to acetonitrile (120 ml), ...
Embodiment 2
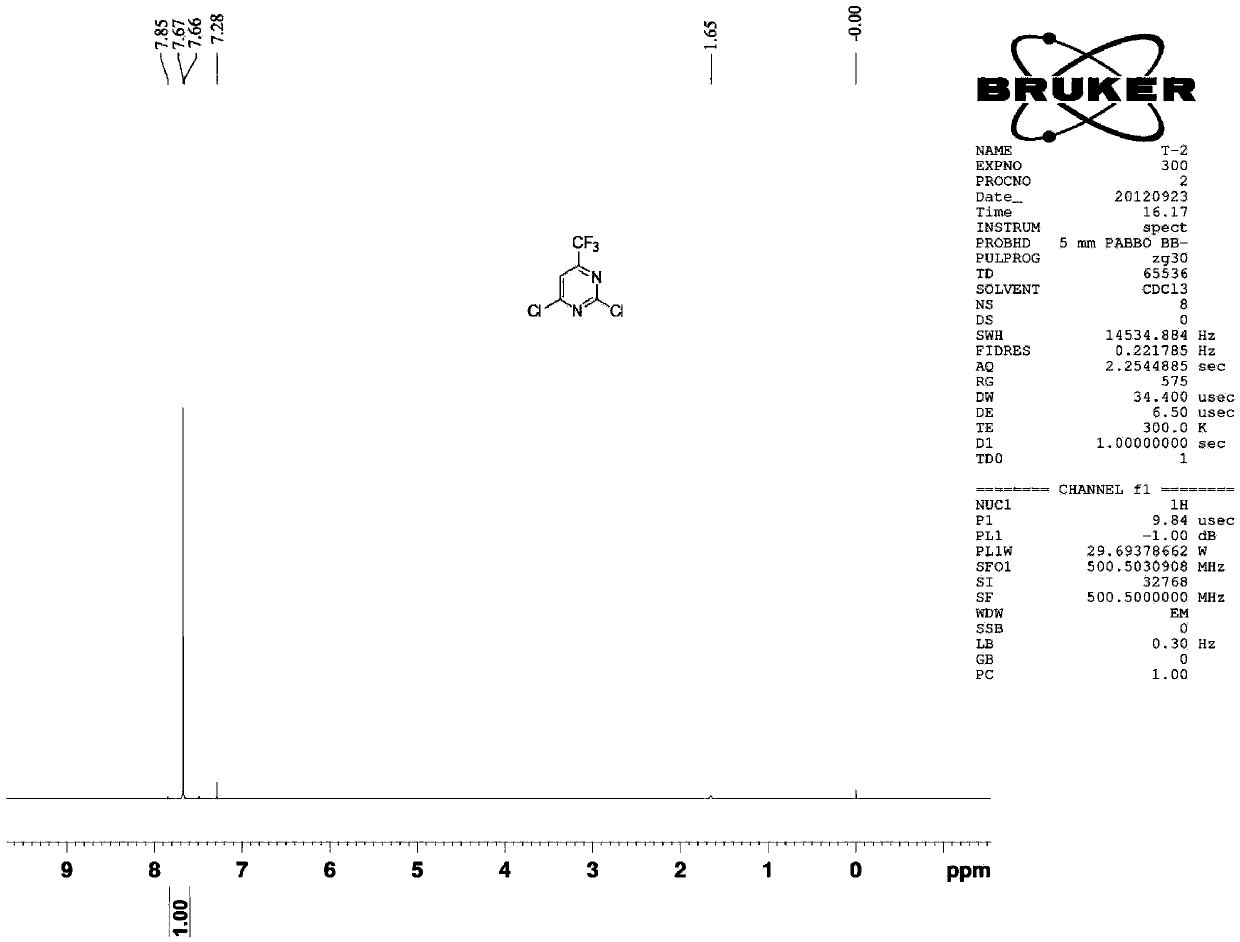
[0036] Example 2 2,4-dichloro-6-trifluoromethylpyrimidine (CAS16097-64-6)
[0037]
[0038] Ethyl trifluoroacetylacetate (16.8 grams, 0.091mol), urea (6.5 grams, 0.109mol), sodium ethylate (24.7 grams, 0.364mol) were added to a mixed solvent composed of 120ml toluene and 40ml ethanol, and the reaction apparatus Add water separator. Raise the reaction temperature from normal temperature to 120°C to 130°C and reflux for 12 hours. During the reaction, use a water separator to separate water from oil to remove water. The reaction solvent was spin-dried, adjusted to neutrality by adding 10% hydrochloric acid, and extracted three times with 80 ml of ethyl acetate. The ethyl acetate phases were combined, dried, filtered and spin-dried to obtain the crude product, which was washed with petroleum ether to obtain 15.2 g of 2,4-dihydroxy-6-trifluoromethylpyrimidine, with a yield of 92.8%.
[0039] 2,4-dihydroxy-6-trifluoromethylpyrimidine (15.7 g, 0.087 mol, 1 eq) was added to aceto...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com