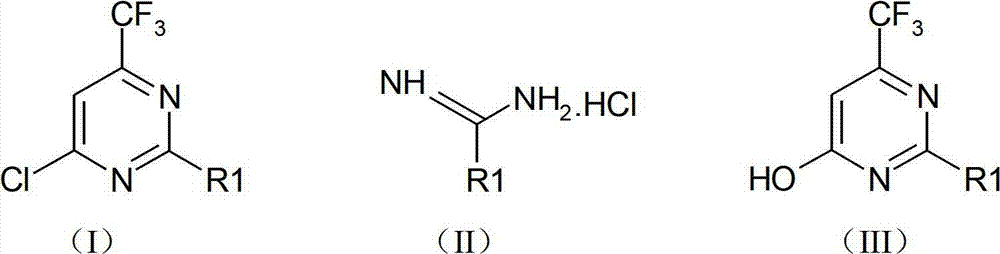
Preparation method of 4-chloro-6-trifluoromethylpyrimidine type compound
A technology of trifluoromethylpyrimidine and compound, applied in the field of organic synthesis, can solve the problems of many by-products, increase yield, low yield and the like, and achieve the effects of easy operation, low cost and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
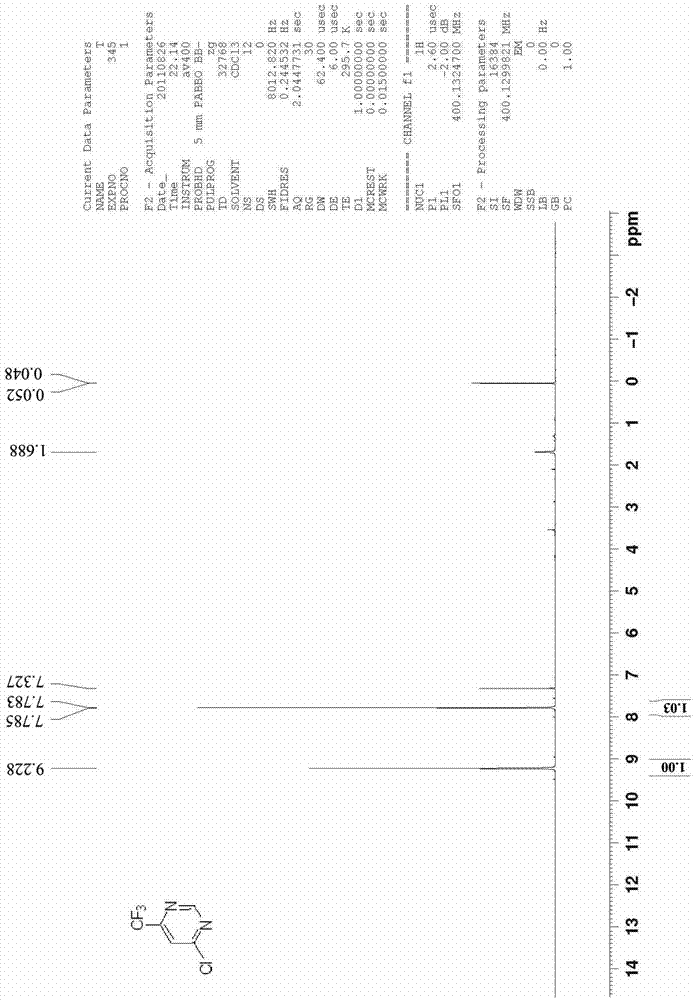
[0026] Example 1 Synthesis of 4-chloro-6-trifluoromethylpyrimidine (CAS 37552-81-1)
[0027]
[0028] Add ethyl trifluoroacetylacetate (15.6 g, 0.085 mol), methyl ether hydrochloride (8.2 g, 0.102 mol), sodium ethoxide (23.1 g, 0.341 mol), into a mixed solvent consisting of 120 ml of toluene and 40 ml of ethanol , and the reaction device plus water separator. Raise the reaction temperature from normal temperature to 120°C-130°C and reflux for 12 hours. The reaction solvent was spin-dried, adjusted to neutrality (pH=6.5-7.5) by adding 10% hydrochloric acid, and extracted three times with 50 ml of ethyl acetate. The ethyl acetate phases were combined, dried, filtered and spin-dried to obtain a crude product; washed with petroleum ether to obtain 13.1 g of 4-hydroxy-6-trifluoromethylpyrimidine, with a yield of 93.6%.
[0029] 4-Hydroxy-6-trifluoromethylpyrimidine (13.1 g, 0.080 mol) was added to acetonitrile (120 ml), and POCl was added under room temperature and stirring 3...
Embodiment 2
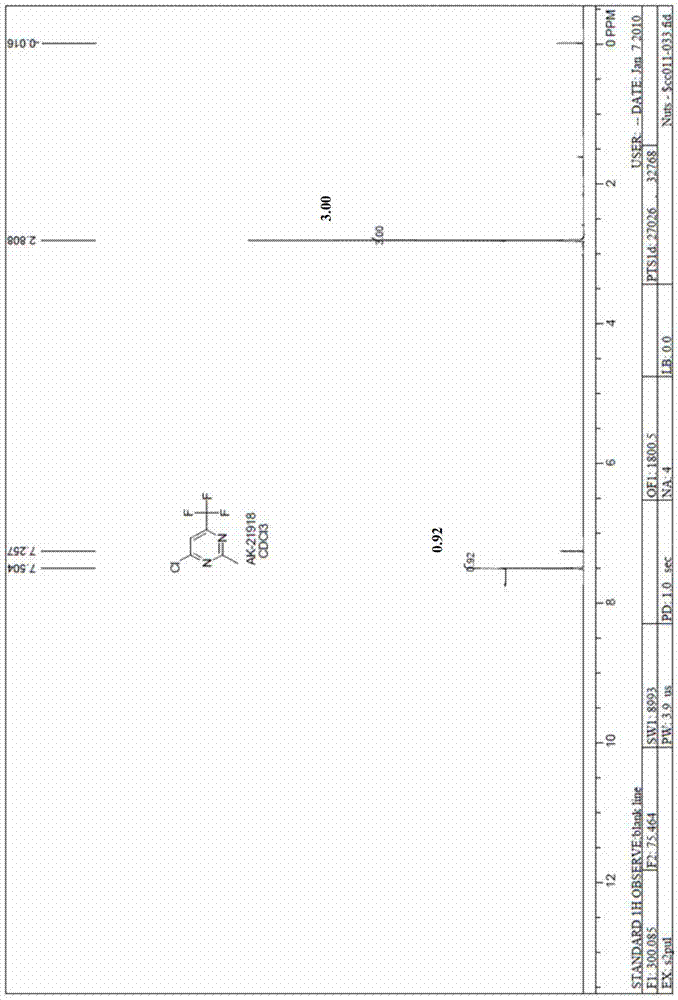
[0032] Example 2 Synthesis of 4-chloro-2-methyl-6-trifluoromethylpyrimidine (CAS 5993-98-6)
[0033]
[0034] Ethyl trifluoroacetylacetate (12.3 grams, 0.067mol), diethyl ether hydrochloride (7.5 grams, 0.080mol), sodium ethylate (18.2 grams, 0.267mol) were added to a mixed solvent of 120ml toluene and 40ml ethanol, and the reaction apparatus Add water separator. Raise the reaction temperature from normal temperature to 120°C to 130°C, reflux for 12 hours, and use a water separator to separate and remove water during the reaction. The reaction solvent was spin-dried, adjusted to neutrality by adding 10% hydrochloric acid, and extracted three times with 50 ml of ethyl acetate. The ethyl acetate phases were combined, dried, filtered and spin-dried to obtain a crude product, which was washed with petroleum ether to obtain 11.4 g of 4-hydroxy-2-methyl-6-trifluoromethylpyrimidine with a yield of 95.7%.
[0035] 4-Hydroxy-6-trifluoromethylpyrimidine (11.4 g, 0.064 mol) was adde...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com