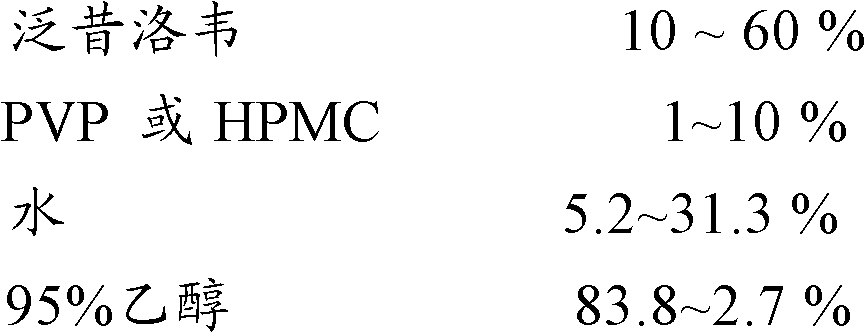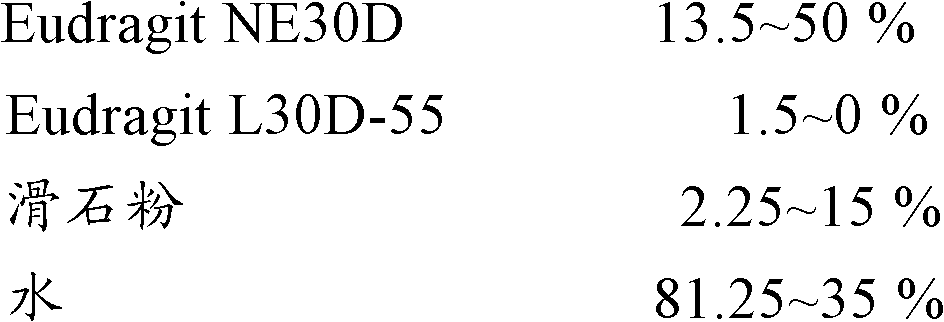Famciclovir sustained-release pellet, preparation method and application thereof
A technology of sustained-release pellets and famciclovir, which is applied in the direction of pharmaceutical formulas, medical preparations with non-active ingredients, and the digestive system. Effects of compliance, reduction of side effects, and reduction of times of taking medication
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] The present embodiment provides in the famciclovir sustained-release pellets, the medicinal solution prescription and preparation method of the main drug layer, specifically as follows:
[0045] Liquid prescription 1:
[0046]
[0047] Use the solution application method to wrap the main drug layer drug solution of the above prescription outside the blank ball core, and the specific operation is as follows:
[0048] Turn on the fluidized bed, send 250g of sucrose pellets (0.4-0.6mm) into the fluidized bed, after adjusting the parameters, put 2700g of the liquid medicine prepared according to the prescription into the fluidized bed by the peristaltic pump, and spray it on medicine, and make medicine pellets. The yield is not less than 90%.
[0049] Liquid prescription 2:
[0050]
[0051] Use the solution application method to wrap the main drug layer drug solution of the above prescription outside the blank ball core, and the specific operation is as follows: ...
Embodiment 2
[0067] The present embodiment provides in the famciclovir sustained-release pellets, the coating liquid prescription of sustained-release layer and preparation method thereof, specifically as follows:
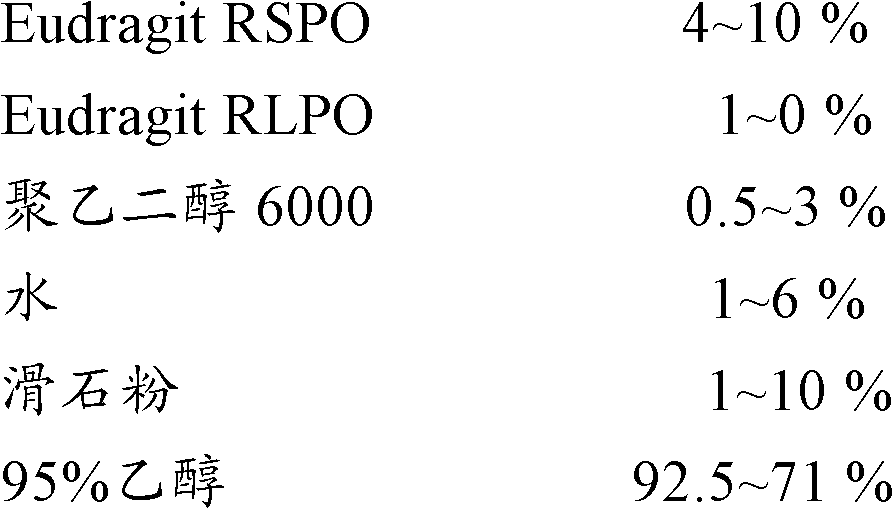
[0068] Coating Solution Prescription 1:
[0069]
[0070] Wrap the coating solutions of the above different prescriptions on the outside of the drug pellets, the specific operation is as follows:
[0071] Turn on the fluidized bed, send 400 g of the famciclovir drug pellets (0.8-0.9 mm) prepared according to the prescription 1 of the traditional Chinese medicine solution in Example 1 into the fluidized bed, spray and coat the coating solution, and carry out in a 40° C. oven after coating Aged for 12h.
[0072] Coating Solution Prescription 2:
[0073]
[0074] Wrap the coating solutions of the above different prescriptions on the outside of the drug pellets, the specific operation is as follows:
[0075] Turn on the fluidized bed, send 400 g of the famciclovir drug pel...
Embodiment 3
[0091] In this example, samples were taken at different coating amounts of polymers during the preparation of sustained-release pellets, and their release rates were measured. The specific results are as follows.
[0092] The release rate is measured with reference to the first method of Appendix XD of the Chinese Pharmacopoeia 2005 edition, the details are as follows. Adopt the device of the first method of dissolution measurement method, with 0.1mol / L hydrochloric acid 750ml as solvent, rotating speed is 100 revolutions per minute, operate according to law, through 1.5 hours, get solution 10ml, filter, as need testing solution 1, in time Add 10ml of 0.1mol / L hydrochloric acid to the operating container, and at the same time add 250ml of 0.2mol / L sodium phosphate solution at 37°C to the 0.1mol / L hydrochloric acid (use 2mol / L hydrochloric acid solution or 2mol / L sodium hydroxide solution if necessary) Adjust the pH value to 6.8 ± 0.05); take 10ml of the solution at 3 hours and...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap