Ginkgolide K preparation and preparation method thereof
A technology for ginkgolides and preparations, which is applied in the field of ginkgolide K preparations and its preparation, can solve the problems of small dosage, poor water solubility of ginkgolide K, slow dissolution rate, etc., and achieve fast release rate, low cost, and fast effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
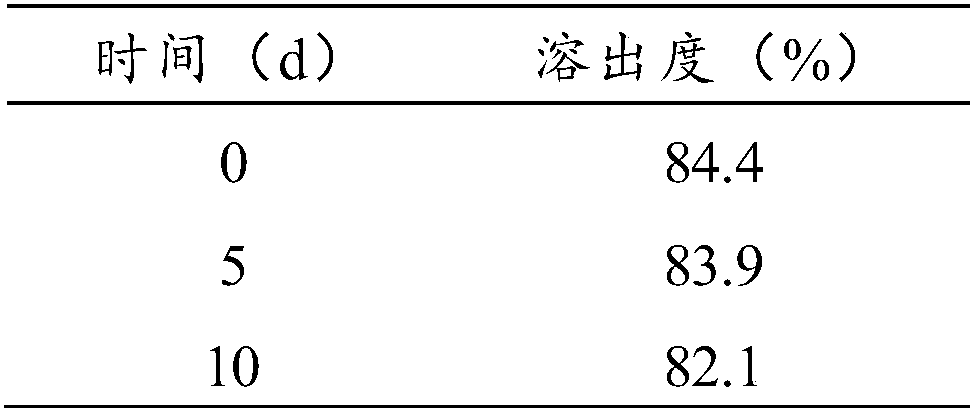
[0029] The present invention also provides a kind of preparation method of ginkgolide K capsule, specifically comprises the following steps:
[0030] Step 1. The ginkgolide K of 7.5~15 mass parts, the hydrophilic polymer carrier of 30~75 mass parts, the solubilizer of 5~40 mass parts and the plasticizer and the plasticizer of 2~15 mass parts are fully mixed standby;
[0031] Step 2. Set the extrusion temperature of the screw extruder, start the screw after the temperature rises to the set value, add the physical mixture in step 1 into the extruder, melt and extrude, and extrude in strips , connect the extrudate to a glass plate, after cooling, set aside;
[0032] Step 3. Pulverize the strips obtained in step 2 to obtain drug solid dispersion granules or powder, sieve, and pack into capsules.
[0033] In some specific embodiments of the present invention, step 3 in the preparation is specifically: crush the strip obtained in step 2 through a 30-80 mesh sieve, and pack it into...
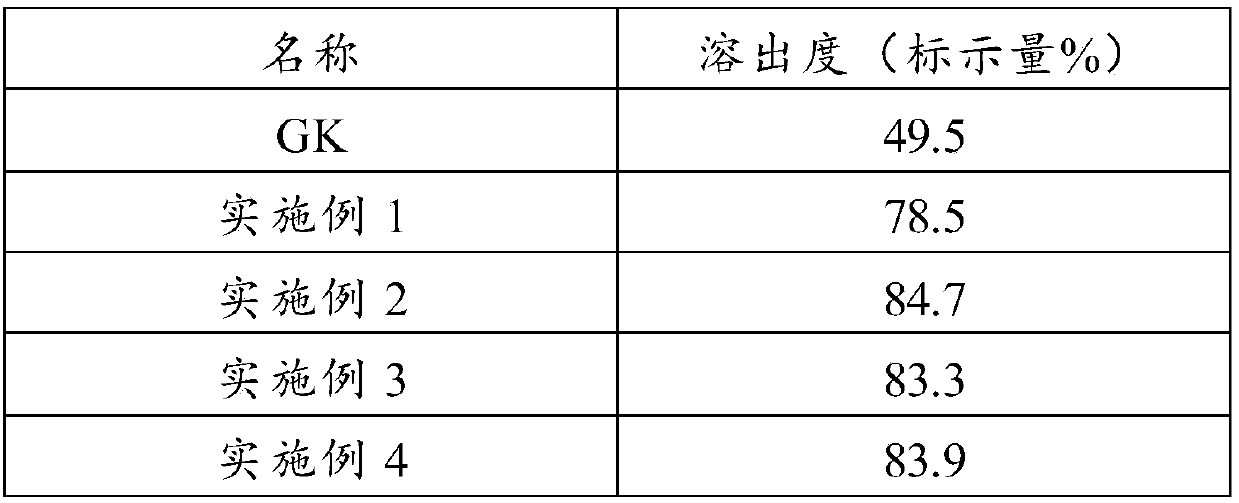
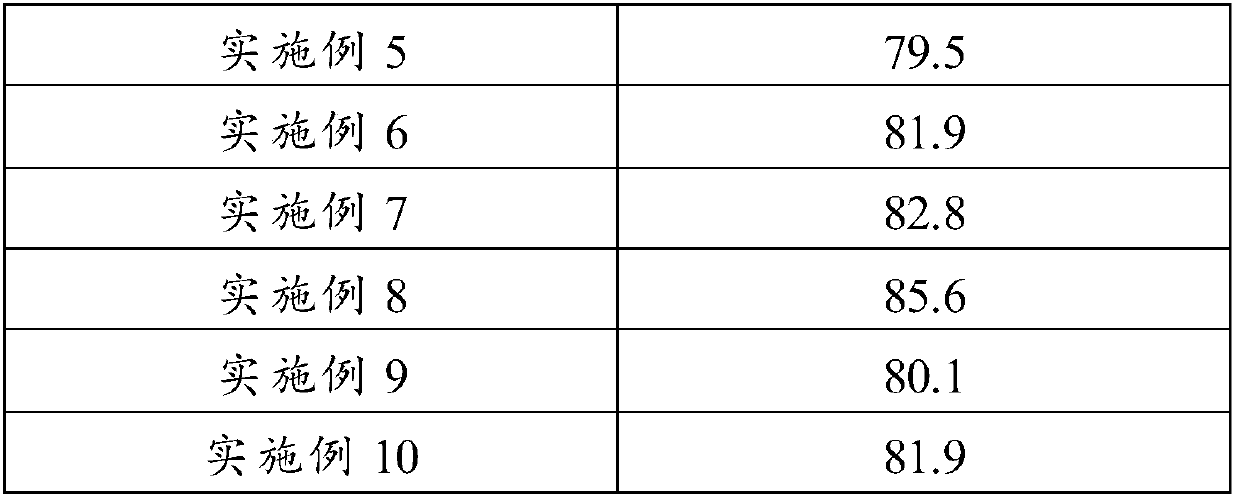
Embodiment 1
[0041] Take 15g of ginkgolide K, 75g of polyethylene glycol 6000, 10g of poloxamer and 5g of citrate and mix them fully and uniformly, put them in the hopper, set the temperature of the extruder at 90-100°C, and wait until the temperature rises to After the set value is stable, keep the feeding rate at 15g / min, and add the mixture at a constant speed to obtain a strip-shaped extrudate, cool at 26°C, crush through a 40-mesh sieve, and fill the capsule.
Embodiment 2
[0043] Take 10g of ginkgolide K, 70g of polyethylene glycol 4000, 10g of polysorbate and 10g of polyethylene glycol and mix them fully and uniformly, put them in the hopper, set the temperature of the extruder at 130-140°C, and wait until the temperature rises to the set temperature. After the value is fixed and stable, keep the feeding rate at 15g / min, and add the mixture at a constant speed to obtain a strip-shaped extrudate, cool at 30°C, crush through a 40-mesh sieve, and fill the capsule.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com