A kind of pharmaceutical composition containing idebenone and its preparation method
A technology of idebenone and its composition, which is applied in the field of pharmaceutical preparations, can solve the problem of low percentage of related substances in grinding, and achieve the effects of good redispersibility, high drug loading, and low content of related substances
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
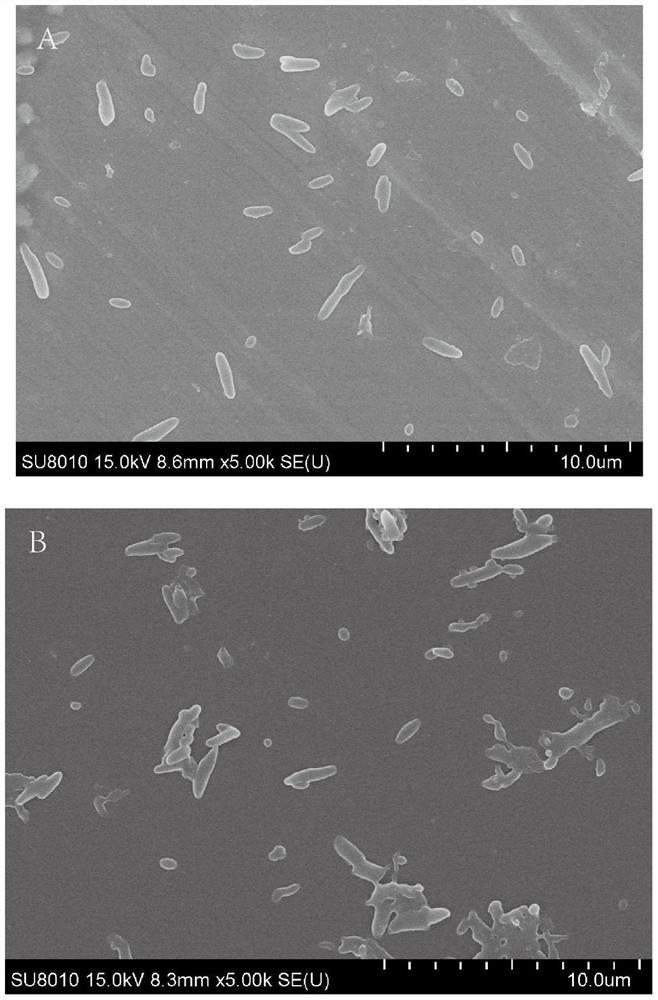
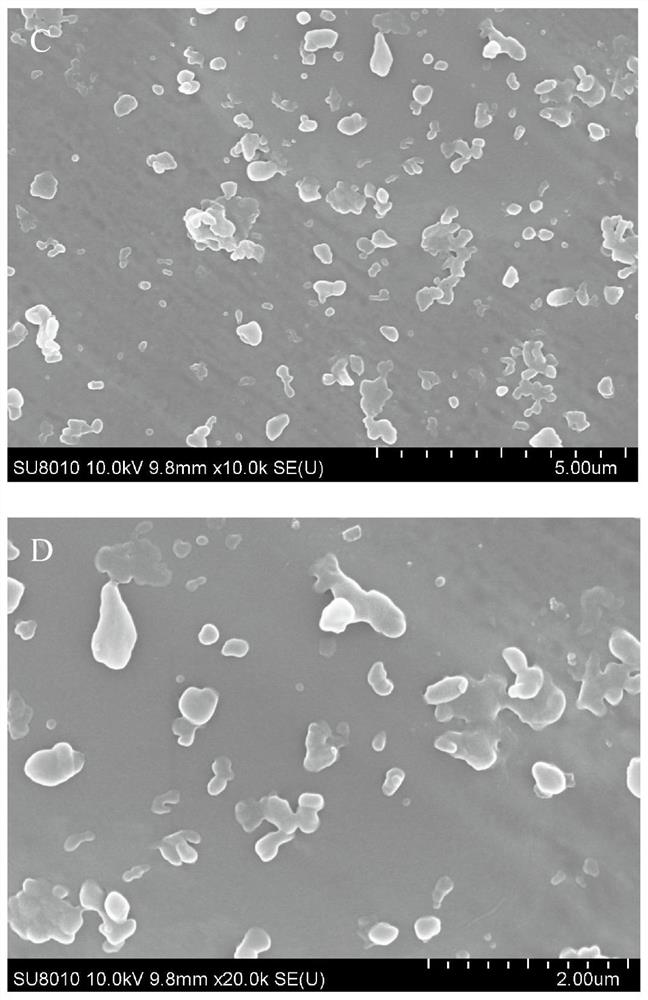
Image
Examples
Embodiment 1
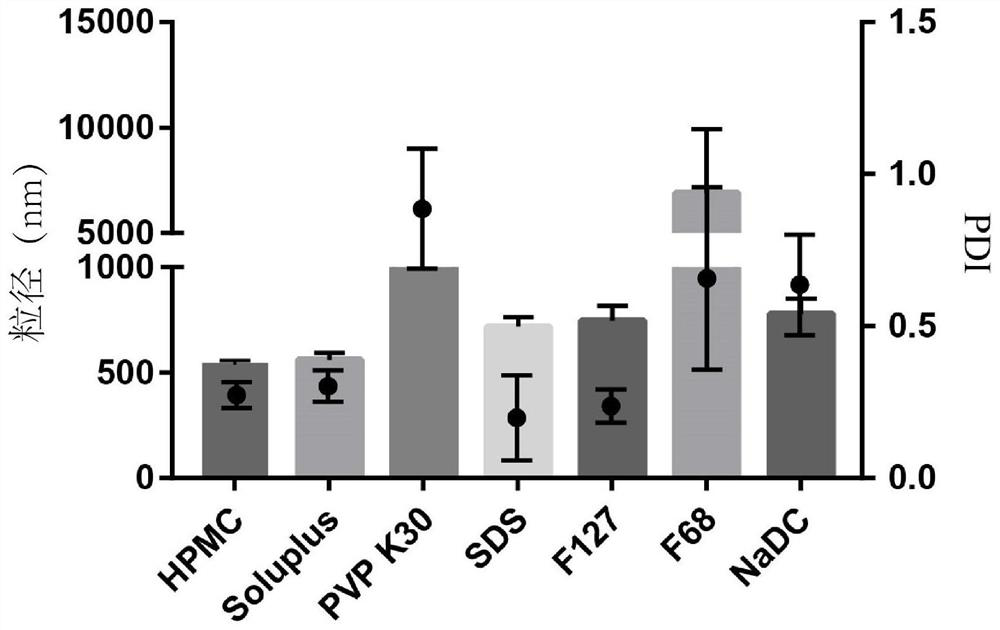
[0048] Example 1: Types and dosage screening of idebenone pharmaceutical composition suspension excipients
[0049] First, prepare polyethylene caprolactam-polyvinyl acetate-polyethylene glycol graft copolymer (Soluplus), hydroxypropylmethylcellulose (HPMC), poloxamer containing 0.2-8.0% (w / v) (F127, F68), sodium deoxycholate (NaDC), sodium dodecyl sulfate (SDS), polyvinylpyrrolidone (PVP), etc., disperse 5% (w / v) idebenone in 20 mL of the above aqueous phase was mixed on a magnetic stirrer for 30 min, then transferred to a 100 mL ball mill jar, and 50 g of 0.4 mm zirconia ball mill balls were added at the same time, and ground for 104 min by a ball mill. Measure the particle size (Z-average) and polydispersity index (PDI) of each prescription as shown in Table 1, when the concentration of auxiliary materials is 1%, the particle size distribution diagrams of various auxiliary materials are as follows: figure 1 shown. The results of the data in Table 1 show that the stabilizi...
Embodiment 2
[0057] Example 2: Preparation process screening of idebenone pharmaceutical composition suspension
[0058] According to the two optimal prescriptions screened out, the grinding process was optimized. Because the grinding power and grinding time have a great influence on the efficiency of sample grinding, too long grinding time will affect the content of related substances and the dispersion state of particles, and the idebenone selected in the present invention has a low melting point, and the preparation method produces More heat, in order to ensure good stability of the prepared pharmaceutical composition, the grinding process is optimized to improve the grinding efficiency.
[0059] Firstly, the total grinding time is screened, the grinding power is fixed at 35Hz, and the particle size distribution is investigated at different grinding times. The results are as follows Figure 4 As shown, the particle size change rate slowed down after grinding to 40min, and the particle ...
Embodiment 3
[0073] Example 3: Preparation and crystal form characterization of a freeze-dried powder of idebenone pharmaceutical composition
[0074] In order to investigate the existing state of the drug in the pharmaceutical composition, the prepared liquid preparation was solidified by using a freeze-drying method. In order to maintain the superior characteristics of the preparation, it is necessary to add a lyoprotectant before lyophilization to maintain the integrity of the nanostructure of the preparation, and the particle size distribution can still be maintained after redispersion, so the type of lyoprotectant was screened.
[0075] First, carry out preliminary screening of the type and concentration of the lyoprotectant, add 2%-10% (w / v) lyoprotectant to the pharmaceutical composition suspension after grinding, the lyoprotectant is selected from maltose ( maltose), lactose, D-mannitol, sucrose, sorbitol, cyclodextrin, dextran and polyethylene glycol (PEG 4000, PEG6000 ). The pa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com