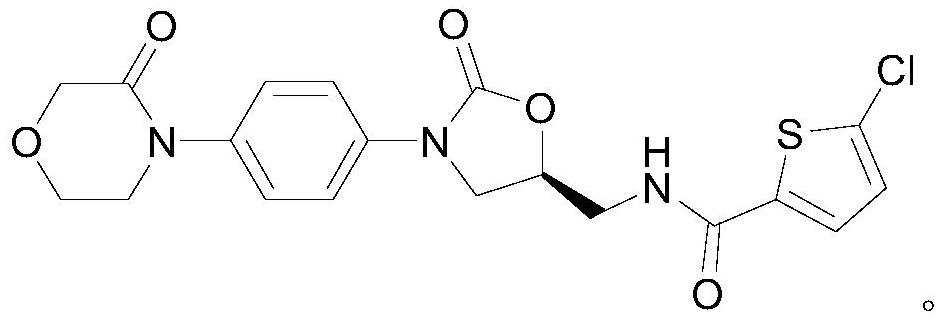
Rivaroxaban tablet and preparation method thereof
A technology for rivaroxaban and tablets, applied in the field of rivaroxaban tablets and their preparation, can solve problems such as complicated process and achieve the effects of requiring simple equipment, stable release and simple components
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Prepare the formulation composition of rivaroxaban according to the formula in Table 1:
[0039] Table 1.
[0040] Dosage(kg) Rivaroxaban (d90=13.8μm) 0.5 Mannitol microcrystalline cross-polysilicone co-treatment 6 Magnesium stearate 0.03 total 6.53
[0041] Preparation method: first mix the rivaroxaban raw material with the same amount of co-processed auxiliary materials and magnesium stearate in the material bag, and then transfer the above mixed powder and the remaining co-processed material to the HLS-50 laboratory hopper In the mixer, set the mixing frequency to 8rpm and the mixing time to 20 minutes for physical mixing, measure the content of the intermediate, control the hardness of the tablet to 50-80N, and compress the intermediate granules.
Embodiment 2
[0043] Prepare the formulation composition of rivaroxaban according to the formula in Table 2:
[0044] Table 2.
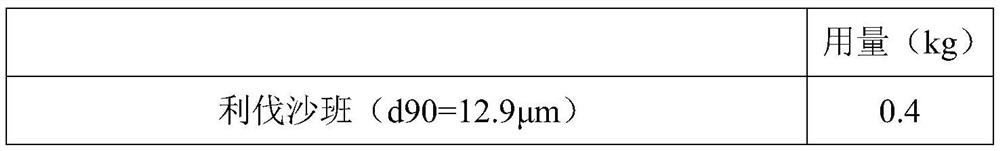
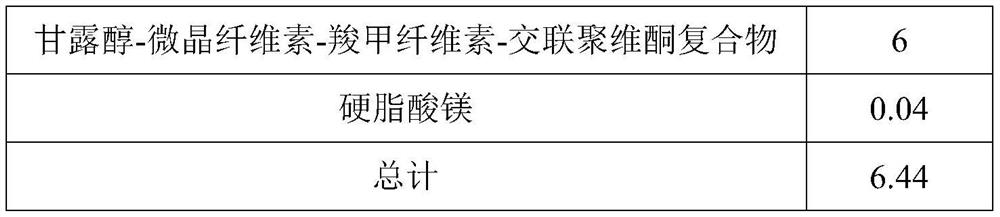
[0045]
[0046]
[0047] Preparation method: first mix the rivaroxaban raw material with the same amount of co-processed auxiliary materials and magnesium stearate in the material bag, and then transfer the above mixed powder and the remaining co-processed material to the HLS-50 laboratory hopper In the mixer, set the mixing frequency to 12 rpm and the mixing time to 12 minutes for physical mixing, measure the content of the intermediate, control the hardness of the plain tablet to 50-80N, and compress the intermediate granules.
Embodiment 3
[0049] Prepare the formulation composition of rivaroxaban according to the formula in Table 3:
[0050] table 3.
[0051] Dosage(kg) Rivaroxaban (d90=12.6μm) 0.3 Mannitol cross-polymerization copolymer Maishan co-processed substance 500 5.4 Magnesium stearate 0.05 total 5.75
[0052] Preparation method: first mix the rivaroxaban raw material with the same amount of co-processed auxiliary materials and magnesium stearate in the material bag, and then transfer the above mixed powder and the remaining co-processed material to the HLS-50 laboratory hopper In the mixer, set the mixing frequency to 15 rpm and the mixing time to 8 minutes for physical mixing, measure the content of the intermediate, control the hardness of the tablet to 50-80N, and compress the intermediate granules.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com