Compound type therapeutic hepatitis B gene vaccine and construction method thereof
A technology of genetic vaccine and construction method, applied in the field of hepatitis B vaccine, can solve the problem of not being able to completely remove intracellular infection virus, etc., and achieve the effect of good intracellular virus and removal of intracellular virus
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
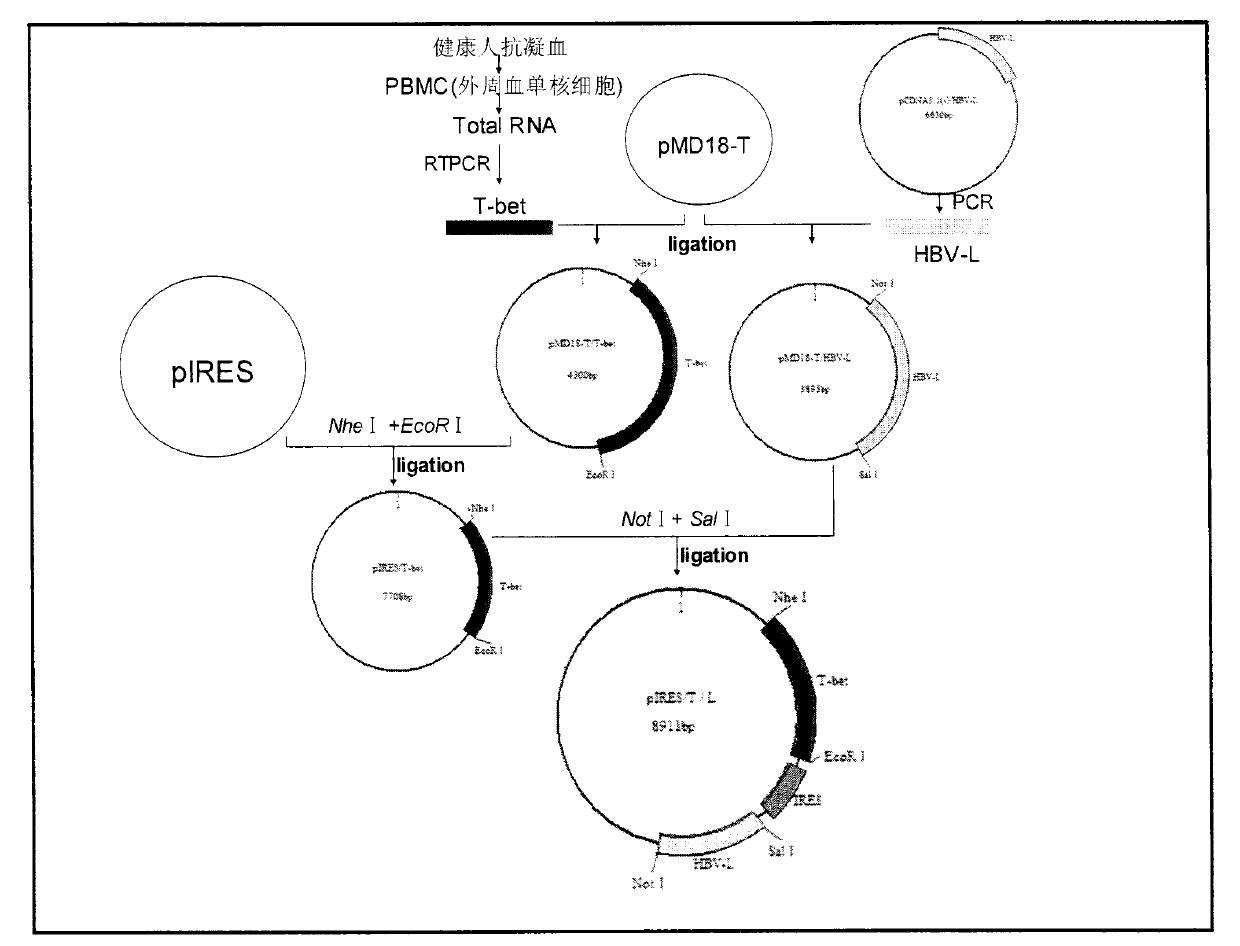
[0025] 1. PCR primer design and synthesis
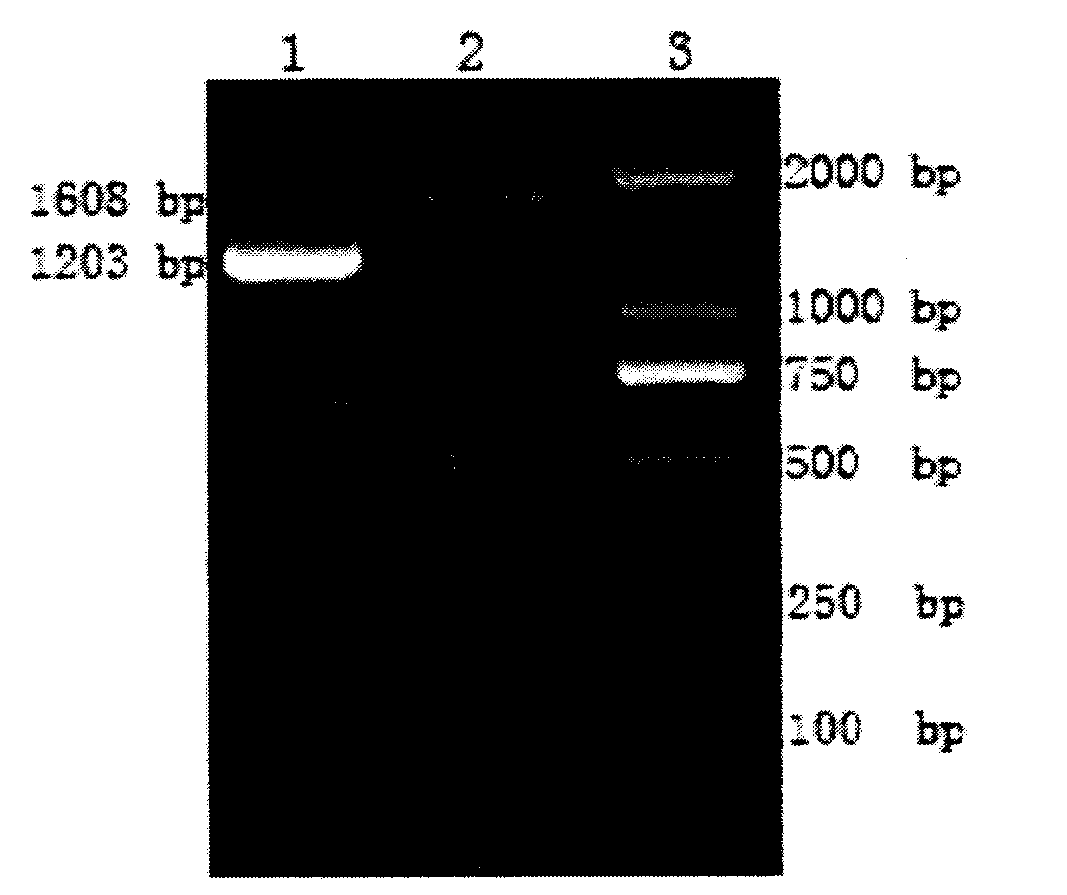
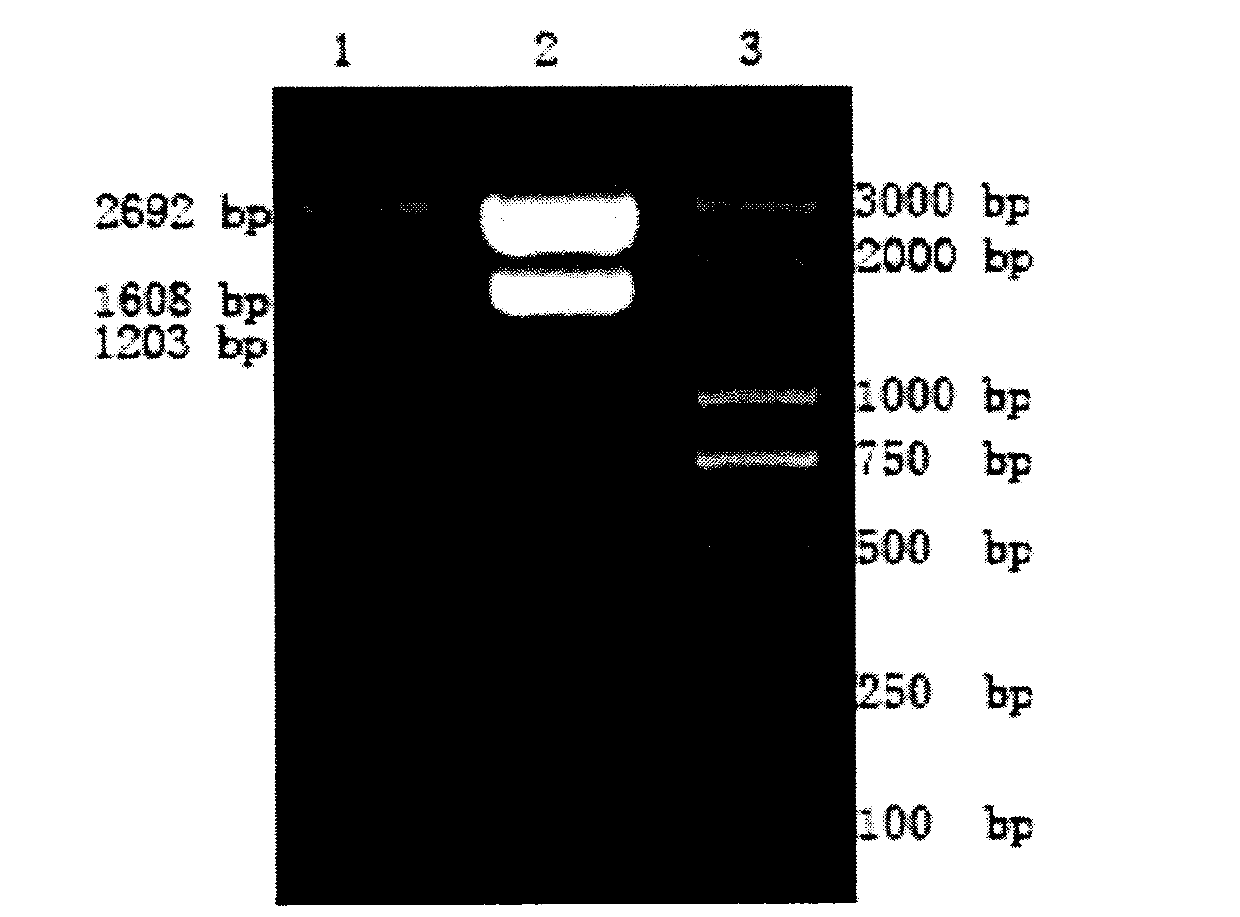
[0026] Two pairs of primers were designed as the primer sequences for amplifying T-bet and HBV-L respectively. See Table 1 for the primer sequences and restriction sites. The sizes of the amplified T-bet and HBV-L gene fragments were 1608bp and 1203bp, respectively. Primers were synthesized by Invitrogen Company.
[0027] Target gene amplification primer sequence
[0028]
[0029] 2. PCR amplification of the target gene
[0030] 2.1 RT-PCR method to clone human T-bet gene
[0031] 2.1.1 Extraction of total RNA from human peripheral blood lymphocytes
[0032] (1) Take 5ml of anticoagulated blood from a healthy person with a blood collection tube, centrifuge at 3000rpm for 5min, and suck out the serum.
[0033] (2) Take a 15ml centrifuge tube and add 2ml Ficoll.
[0034] (3) Add 1ml PBS to the blood collection tube to resuspend the cells, attach to the wall at 45 degrees and add to a centrifuge tube equipped with Ficoll, and c...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap