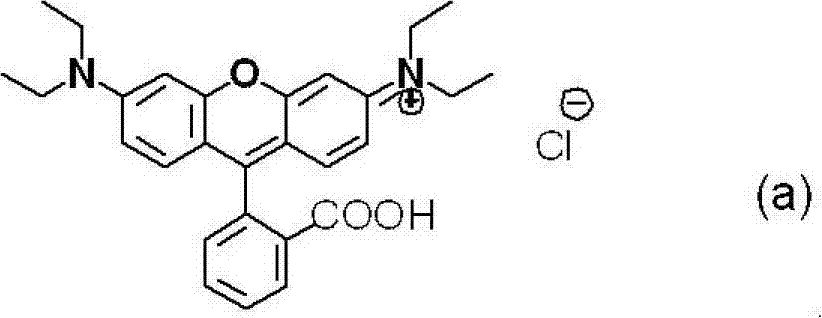
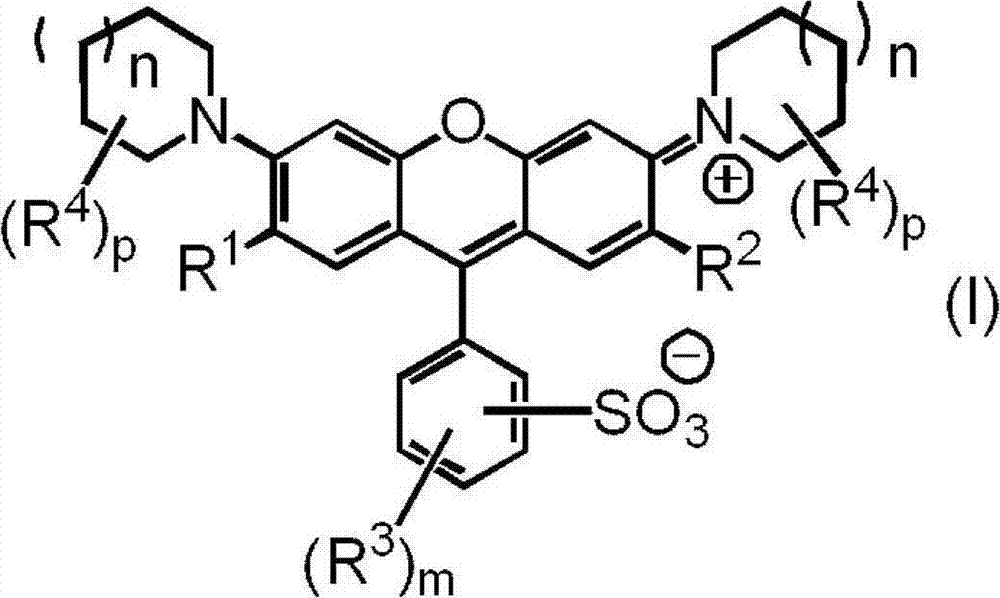
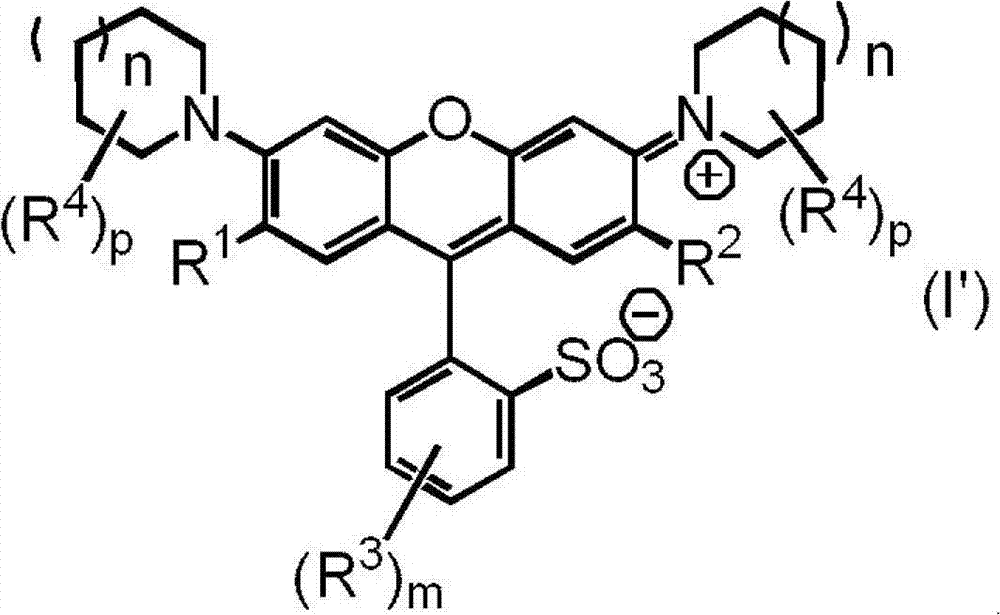
Compound used in color composition
A technology of compound and carbon number, applied in the direction of organic chemistry, chemical instruments and methods, photosensitive materials used in optomechanical equipment, etc., to achieve the effect of excellent solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0135] 12.0 parts of a compound represented by the following formula (II-1), 60.0 parts of N-methyl-2-pyrrolidone, and 12.6 parts of piperidine (manufactured by Tokyo Chemical Industry Co., Ltd.) were mixed, and the resulting mixture was stirred at 60°C for 5 Hour. After cooling the said reaction liquid to room temperature, it added to the liquid mixture of 600 parts of water and 100 parts of 35% hydrochloric acid, and stirred at room temperature for 1 hour. The precipitated crystals were collected as suction-filtered residues and dried to obtain 12.4 parts of the compound represented by the formula (I-1). The yield was 83%.
[0136]
[0137] Identification of compounds represented by formula (I-1)
[0138] (Mass Spectrometry) Ionization Mode = ESI+: m / z = [M+H] + 503.4
[0139] Exact mass: 502.2
Embodiment 2
[0141] Mix 12.0 parts of the compound represented by formula (II-1), 60.0 parts of N-methyl-2-pyrrolidone, and 14.7 parts of 2-methylpiperidine (manufactured by Tokyo Chemical Industry Co., Ltd.), and heat the resulting mixture at 60°C Stirring was continued for 5 hours. After cooling the said reaction liquid to room temperature, it added to the liquid mixture of 600 parts of water and 100 parts of 35% hydrochloric acid, and stirred at room temperature for 1 hour. The precipitated crystals were collected as suction-filtered residues and dried to obtain 13.8 parts of the compound represented by the formula (I-13). The yield was 88%.
[0142]
[0143] Identification of compounds represented by formula (I-13)
[0144] (Mass Spectrometry) Ionization Mode = ESI+: m / z = [M+H] + 531.2
[0145] Exact mass: 530.2
[0146]
[0147] According to the following method, the compounds obtained respectively in Examples 1 and 2 and Rhodamine B (manufactured by Tokyo Chemical Industry...
Embodiment 3
[0154] [Preparation of coloring composition]
[0155] (A) Colorant: Compound (I-1): 20 parts of the compound obtained in Example 1
[0156](B-1) Resin: methacrylic acid / benzyl methacrylate copolymer (molar ratio: 30 / 70; weight average molecular weight 10700, acid value 70mgKOH / g) 70 parts
[0157] (C-1) Polymeric compound: 30 parts of dipentaerythritol hexaacrylate (manufactured by Nippon Kayaku Co., Ltd.)
[0158] (D-1) Photopolymerization initiator: 15 parts of benzyl dimethyl ketal (イルガキュア (registered trademark) 651; manufactured by BASF Japan Co., Ltd.)
[0159] (E-1) Solvent: 680 parts of dimethylformamide
[0160] The above-mentioned substances were mixed to obtain a coloring composition.
PUM
| Property | Measurement | Unit |
|---|---|---|
| acid value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com