Synthesis method for medicine entecavir for treating hepatitis B
A technology of entecavir and medicine, which is applied in the field of synthesizing entecavir, can solve the problems of excessive tar, affecting yield and product purification, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
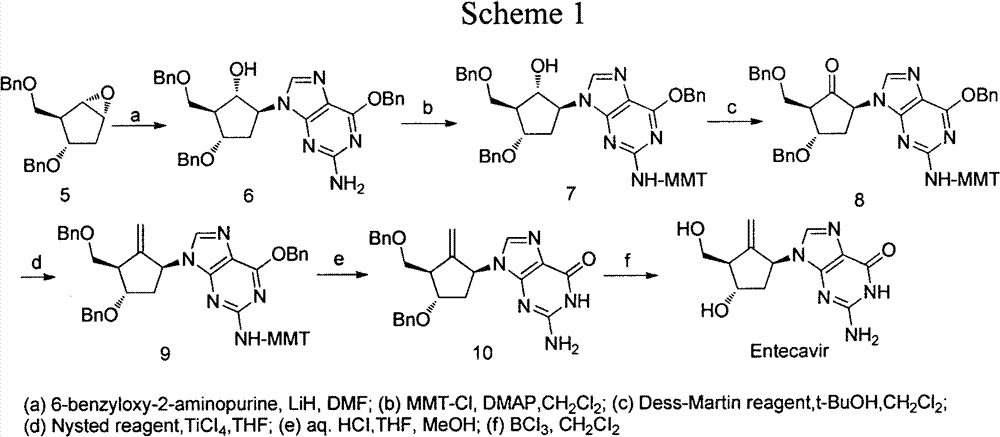
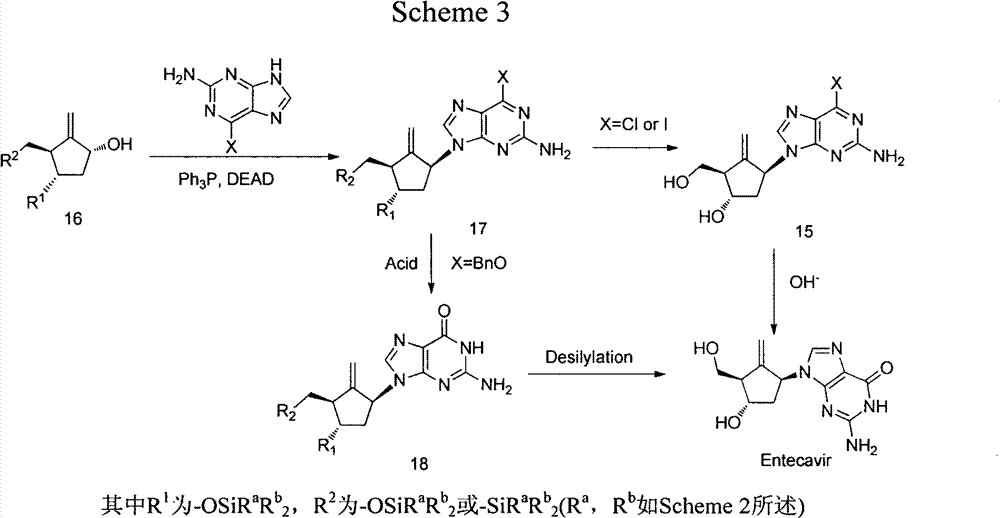
[0019] Example 1. (1S, 2S, 3S, 5S)-5-(2-amino-6-(benzyloxy)-9hydro-purine)-1-hydroxyl-3-(1-phenylcyclotrisilyl) ) Synthesis of cyclopentane-1,2-dimethanol (2a)
[0020] Add O-6-benzylguanine (2.76g, 11.45mmol), lithium hydroxide (0.23g, 9.54mmol), DMF (21mL) in 50mL one-necked flask, stir for 30min, add (1R, 2S, 3S, 5R )-3-(1-phenylcyclotrisilyl)-6-oxabicyclo[3.1.0]hexane-1,2-dimethanol (1a) (2.77g, 9.54mmol), heated to 80°C, React for 24 hours, add 50 mL of water, stir for 10 minutes, extract with ethyl acetate, wash the organic phase with saturated brine, dry over anhydrous sodium sulfate, evaporate the solvent under reduced pressure, and recrystallize from ethyl acetate / petroleum ether to obtain (1S, 2S , 3S, 5S)-5-(2-amino-6-(benzyloxy)purine)-1-hydroxyl-3-(1-phenylcyclotrisilyl)cyclopentane-1,2-dimethanol ( 2a) (3.39 g, yield: 67%).
Embodiment 2
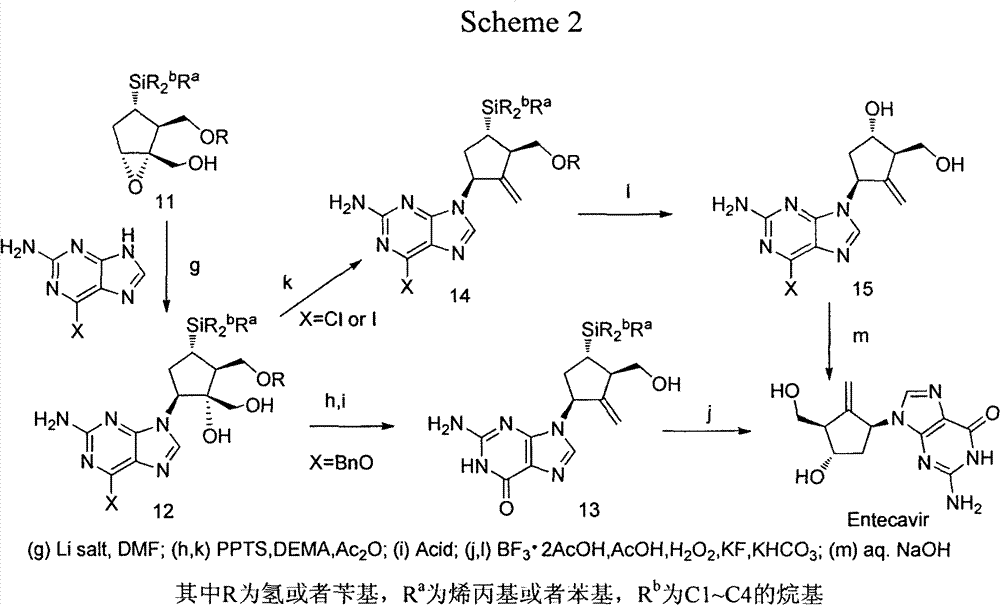
[0021] Example 2. (1S, 2S, 3S, 5S)-5-(2-amino-6-(benzyloxy)-9hydro-purine)-1-hydroxyl-3-(1-phenylcyclotetrasilyl) ) Synthesis of cyclopentane-1,2-dimethanol (2b)
[0022] (1R, 2S, 3S, 5R)-3-(1-phenylcyclobutasilyl)-6-oxabicyclo[3.1.0]hexane-1,2-dimethanol (1b) under the catalysis of lithium hydroxide, React with 6-benzylguanine according to the operation steps of Example 1 to obtain (1S, 2S, 3S, 5S)-5-(2-amino-6-(benzyloxy)-9 hydrogen-purine)-1- Hydroxy-3-(1-phenylcyclotetrasilyl)cyclopentane-1,2-dimethanol (2b), yield: 66%, mp=54-56°C, 1 HNMR (500MHz, DMSO-d 6 )δ: 7.87(s, 1H), 7.59-7.58(m, 2H), 7.51-7.49(m, 2H), 7.39-7.32(m, 6H), 6.47(s, 2H), 5.48(t, 2H, J=12.55, 14.1), 5.05(t, 1H, J=5.8), 4.97(s, 1H), 4.75(t, 1H, J=4.6), 4.48(t, 1H, J=9.8), 3.58-3.52 (m, 2H), 3.35(m, 1H), 3.24(m, 1H), 2.70(m, 1H), 2.09-2.02(m, 2H), 1.84-1.78(m, 1H), 1.69-1.60(m , 4H), 0.98-0.83 (m, 4H).
Embodiment 3
[0023] Example 3. (1S, 2S, 3S, 5S)-5-(2-amino-6-(benzyloxy)-9hydro-purine)-1-hydroxy-3-(1-phenylcyclopentasilyl) ) Synthesis of cyclopentane-1,2-dimethanol (2c)
[0024] (1R, 2S, 3S, 5R)-3-(1-phenylcyclopentasilyl)-6-oxabicyclo[3.1.0]hexane-1,2-dimethanol (1c) was catalyzed by lithium hydroxide Next, react with 6-benzylguanine according to the operation steps of Example 1 to obtain (1S, 2S, 3S, 5S)-5-(2-amino-6-(benzyloxy)-9 hydrogen-purine)- 1-Hydroxy-3-(1-phenylcyclopentasilyl)cyclopentane-1,2-dimethanol (2c), yield: 69%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com