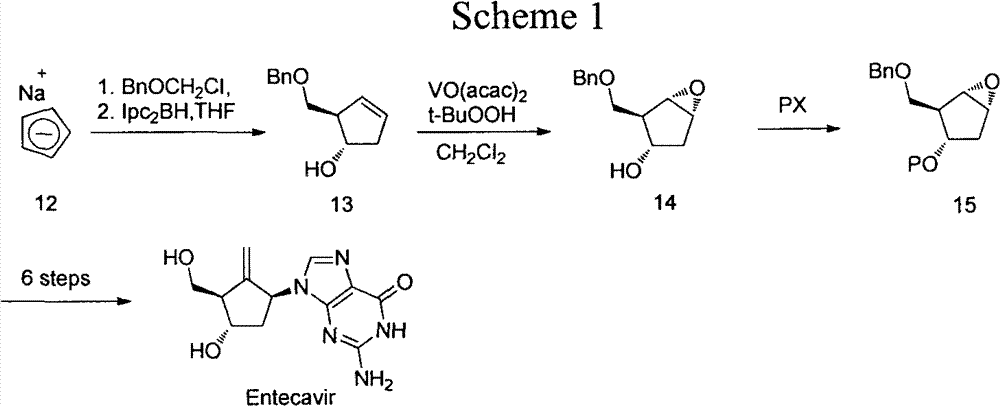
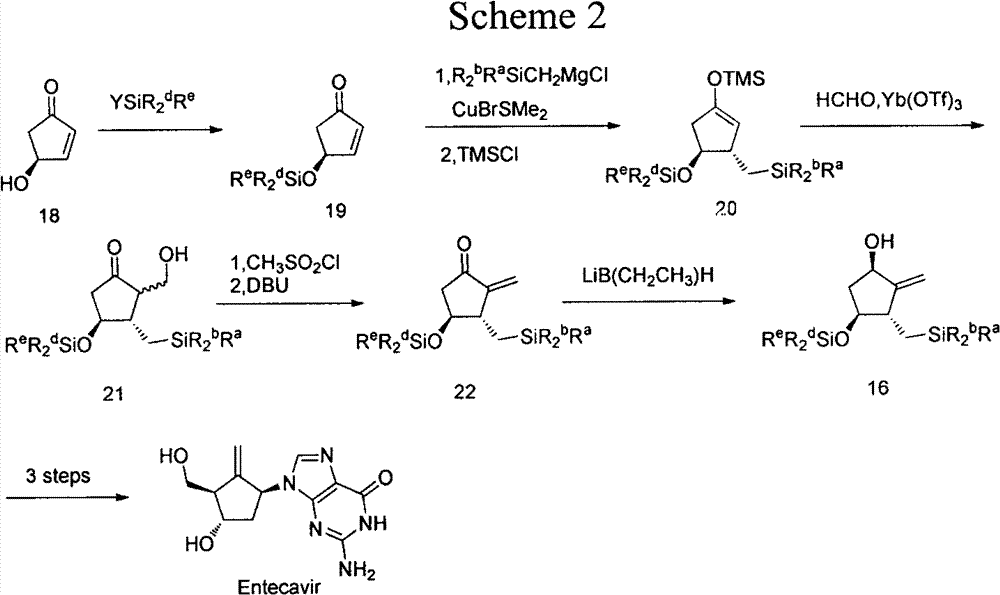
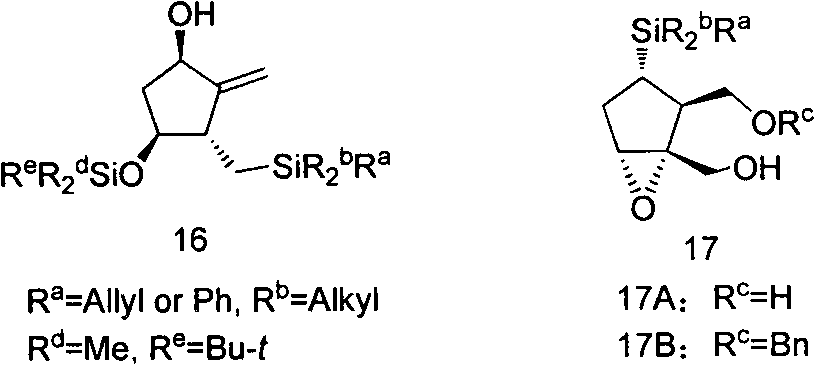Anti-hepatitis B drug entecavir intermediate and synthesis thereof
A technology for entecavir and anti-hepatitis B, which is applied to the intermediate of entecavir and its synthesis field, and can solve the problems of long synthesis steps, difficulty in product purification, low economic benefits and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 11
[0030] Embodiment 1.1, the synthesis of 1-diphenylcyclotrisilane (3a)
[0031] Under nitrogen protection, magnesium chips (53g, 2.21mol) and anhydrous tetrahydrofuran (1400mL) were added to a 2L dry four-neck flask, heated to reflux, and 1,3-dibromopropane (202g, 1.01mol) was slowly added dropwise, After dropping within 2h, continue to reflux for 2h, remove the oil bath, cool to 0°C, add diphenyldichlorosilane (2) (240g, 0.92mol) dropwise, drop within 2h, reflux for 24h, cool down To 0°C, quench the reaction with saturated ammonium chloride solution, extract with petroleum ether, wash the organic phase with saturated brine, dry over anhydrous sodium sulfate, concentrate, and distill under reduced pressure to obtain 1,1-diphenylcyclotrisilane (3a) (148.38g, yield: 70%, bp.=110°C-114°C / 2mmHg).
Embodiment 21
[0032] Embodiment 2.1, the synthesis of 1-diphenylcyclotetrasilane (3b)
[0033] Diphenyldichlorosilane (2) and Grignard reagent BrMg(CH 2 ) 4 MgBr was reacted according to the operation steps of Example 1 to obtain 1,1-diphenylcyclotetrasilane (3b), yield: 79%, bp.=124°C-127°C / 3mmHg, 1 H-NMR (300MHz, CDCl 3 )δ: 7.55-7.52 (m, 4H), 7.36-7.23 (m, 6H), 1.82-1.77 (m, 4H), 1.14-1.09 (m, 4H).
Embodiment 31
[0034] Embodiment 3.1, the synthesis of 1-diphenylcyclopentasilane (3c)
[0035] Diphenyldichlorosilane (2) and Grignard reagent BrMg(CH 2 ) 5 MgBr was reacted according to the operation steps of Example 1 to obtain 1,1-diphenylcyclopentasilane (3c), yield: 81%, bp.=133°C-136°C / 3mmHg.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com