Preparation method of five-membered cyclic sulphate
A technology of cyclic sulfate and cyclic sulfite, which is applied in the field of preparation of five-membered cyclic sulfate, can solve the problems of easy generation of metals, many side reactions, expensive reagents, etc., and achieves easy control and high product yield. , the effect of avoiding the use of chlorine-containing reagents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
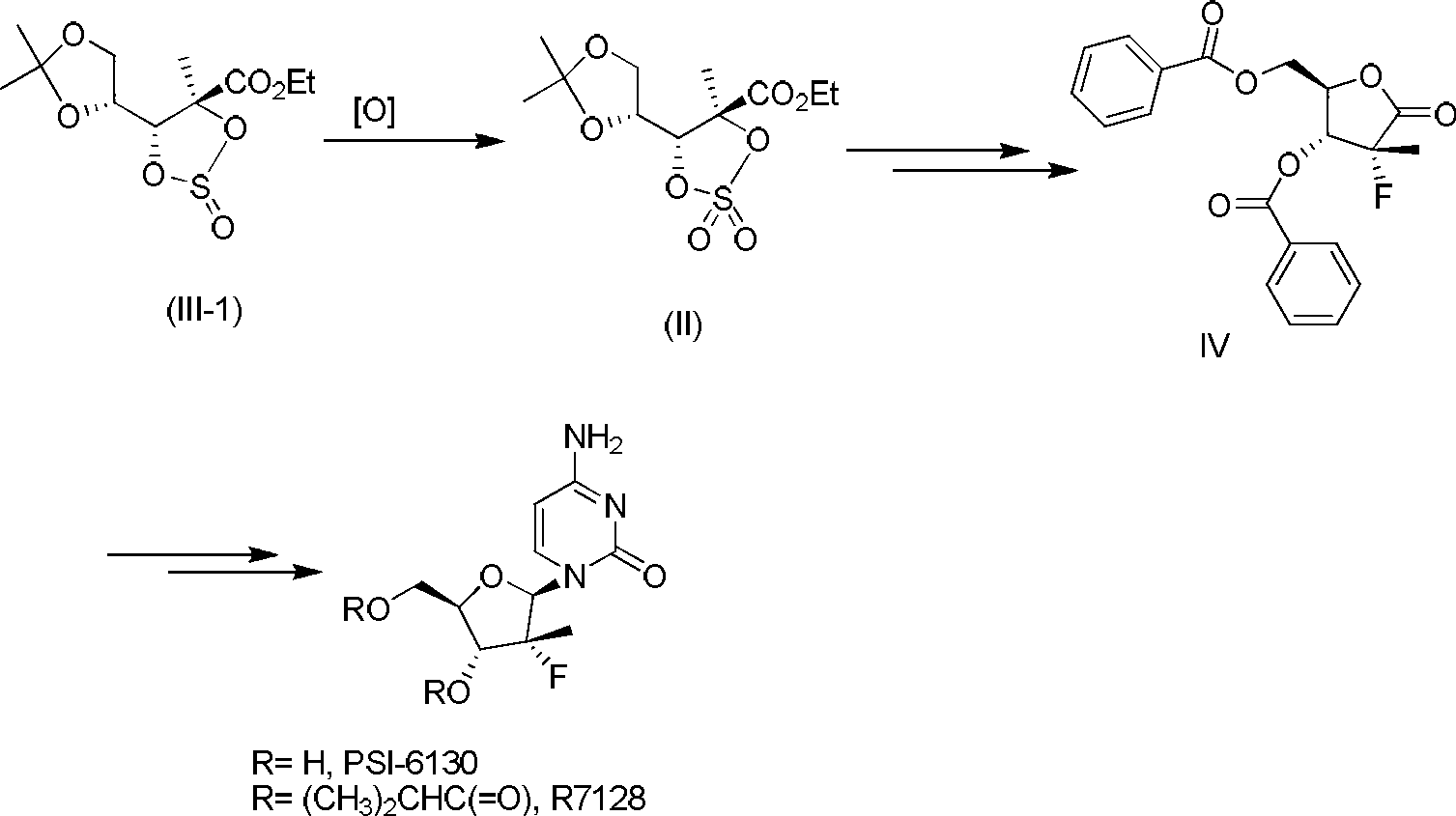
[0035] In this example, OXONE was used as the oxidant, and (2S,3R,4R)-4,5-O-isopropylidene-2,3-cyclosulfinyl-2,3,4,5-tetrahydroxy -Ethyl 2-methylpentanoate (abbreviated as sulfite, according to the method in Wang, P.; Chun, B.; Rachakonda, S. et al. J. Org. Chem. 2009, 74, 6819-6824. prepared) as a raw material, sodium bicarbonate as an additive, and OXONE as an oxidizing agent, wherein OXONE is dissolved in an appropriate amount of water and added, and the amount of water has little influence and can be dissolved. The feeding amount is as follows: sulfite 5g (17mmol), 1,4-dioxane 25mL, sodium bicarbonate 7.5g (89mmol), OXONE 17.8g (29mmol), the order of addition is raw material sulfite, 1,4- Dioxane, sodium bicarbonate, and OXONE aqueous solution, among them, the OXONE aqueous solution was added slowly, and the addition was completed in about 20 minutes. The reaction temperature was 85°C, and the reaction pH was about 6.0. The reaction was quenched with 15 mL of saturated aq...
Embodiment 2
[0037]The raw materials and reagents used in this example are the same as those in Example 1. The feeding amount is as follows: sulfite 5g (17mmol), 1,4-dioxane 25mL, sodium bicarbonate 7.2g (85mmol), OXONE 17.8g (29mmol), the order of addition is raw material sulfite, 1,4- Dioxane, sodium bicarbonate, and OXONE aqueous solution, among them, the OXONE aqueous solution was added slowly, and the addition was completed in about 20 minutes. The reaction temperature was 85°C, and the reaction pH was about 6.0. The reaction was quenched with 15 mL of saturated aqueous sodium bisulfite solution, and extracted with 100 mL of isopropyl acetate. Concentrate after adding diisopropylethylamine to give 4.59g of crude product, yield 87%, GC purity 96%, EI: m / z310.1 (M + ), the effect is better.
Embodiment 3
[0039] The raw materials and reagents used in this example are the same as those in Example 1. The feeding amount is as follows: sulfite 5g (17mmol), 1,4-dioxane 25mL, sodium bicarbonate 7.7g (92mmol), OXONE 17.8g (29mmol), the order of addition is raw material sulfite, 1,4- Dioxane, sodium bicarbonate, and OXONE aqueous solution, among them, the OXONE aqueous solution was added slowly, and the addition was completed in about 20 minutes. The reaction temperature was 85°C, and the reaction pH was about 6.4. The reaction was quenched with 15 mL of saturated aqueous sodium bisulfite solution, and extracted with 100 mL of isopropyl acetate. Concentrate after adding diisopropylethylamine to obtain 4.80 g of crude product, yield 91%, GC purity 96%, EI: m / z 310.1 (M + ), the effect is better.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com